Abstract
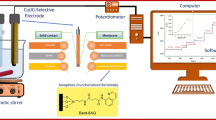
A new modified carbon paste electrode for determination of Cu2+ made in our laboratory that used a new synthesized macrocycle 7,16-diaza-1-thia-4,10,13,19-tetraoxa-6,17-dioxo-2,3;20,21-dinaphtho-cyclouneicosane as modifier. This sensor exhibits a good affinity toward copper (II) ions over a wide variety of other metal ions. The electrode exhibits a Nernstian slope of 30 (±0.5) mV per decade for copper (II) ions over a wide concentration range (1.0 × 10−8–1.0 × 10−2 mol L−1), with a limit of detection of 7.0 × 10−9 mol L−1 (~0.45 ppb). It has a response time of 30 s and can be used for at least 3 months without any considerable divergence in responses. The potentiometric response of the electrode is independent of the pH of test solution in the pH range 3.5–7.5. Finally, it was successfully used as an indicator electrode for determination of copper (II) in real samples such as Karoun river and tap water.






Similar content being viewed by others
References
Mayr, T., Klimant, I., Wolfbeis, O.S., Werner, T.: Dual lifetime referenced optical sensor membrane for the determination of copper(II) ions. Anal. Chim. Acta 462, 1–10 (2002)
Gupta, V.K., Jain, A.K., Maheshwari, G., Langb, H., Ishtaiwi, Z.: Copper (II)-selective potentiometric sensors based on porphyrins in PVC matrix. Sens. Actuators B 117, 99–106 (2006)
Yamini, Y., Hassan, J., Karbasi, M.H.: Solid-phase extraction of copper with cupron on octadecyl silica cartridge and its determination with atomic absorption. Microchim. Acta 148, 305–309 (2004)
Mahendra, N., Gangaiya, P., Subramaniam, S., Narayanaswamy, R.: Investigation of a fibre optic copper sensor based on immobilized a-benzoinoxime (cupron). Sens. Actuators B 90, 118–123 (2003)
Xiang, Y., Tong, A.J., Jin, P.Y., Ju, Y.: New fluorescent rhodamine hydrazone chemosensor for Cu(II) with high selectivity and sensitivity. Org. Lett. 8, 2863–2866 (2006)
Koplik, R., Mestek, O., Fingerora, H., Suchanek, M.: Validation protocol for the determination of copper in plant samples by isotope dilution inductively coupled plasma mass spectrometry. J. Anal. Atom. Spectrom. 14, 241–245 (1999)
Taher, M.A., Mobarakeh, S.Z.M., Mohadesi, A.R.: Determination of trace copper by FAAS after solid phase extraction and preconcentration onto amberlite XAD-2 loaded with nitroso-R salt. Turk. J. Chem. 29, 17–26 (2005)
Ghasemi, J., Shahabadi, N., Seraji, H.R.: Spectrophotometric simultaneous determination of cobalt, copper and nickel using nitroso-R-salt in alloys by partial least squares. Anal. Chim. Acta 510, 121–126 (2004)
Pacheco, W.F., Miguel, E.M., Ramos, G.V., Cardoso, C.E., Farias, P.A.M., Aucélio, R.Q.: Use of hydrogen peroxide to achieve interference-free stripping voltammetric determination of copper at the bismuth-film electrode. Anal. Chim. Acta 625, 22–27 (2008)
Izatt, R.M., Bradshaw, J.S., Nielsen, S.A., Lamb, J.D., Christensen, J.J., Sen, D.: Thermodynamic and kinetic data for cation-macrocycle interaction. Chem. Rev. 85, 271–339 (1985)
Izatt, R.M., Pawlak, K., Bradshow, J.S., Bruening, R.L.: Thermodynamic and kinetic data for macrocycle interaction with cations and anions. Chem. Rev. 91, 1721–2085 (1991)
Faulkner, S., Kataky, R., Parker, D., Teasdale, A.: Lithium selective ionophores based on pendant arm substituted crown ethers. J. Chem. Soc., Perkin Trans. 2, 1761–1770 (1995)
Kimura, K., Shono, T.: Cation Binding by Macrocycles. In: Inoue Y., Gokel G.W. (eds.) chap. 10. Marcel Dekker, New York (1990)
Umezawa, Y.: CRC Handbook of Ion-Selective Electrodes. CRC Press, Boca Raton, FL (1990)
Kalcher, K., Kaufmann, J.M., Yang, Z.: Sensors based on carbon paste in electrochemical analysis: a review with particular emphasis on the period. Electroanalysis 7, 5–22 (1995)
Yeom, J.S., Won, M.S., Shim, Y.B.: Voltammetric determination of the iodide ion with quinine copper (II) complex modified carbon paste electrode. J. Electroanal. Chem. 463, 16–23 (1999)
Hamidi, H., Shams, E., Yadollahi, B., Esfahani, F.K.: Fabrication of bulk-modified carbon paste electrode containing α-PW12O40 3− polyanion supported on modified silica gel: preparation, electrochemistry and electrocatalysis. Talanta 74, 909–914 (2008)
Mousavi, M.F., Rahmanifar, S.M., Golabi, M., Shamsipur, M., Sharghi, H.: Differential pulse anodic stripping voltammetric determination of lead(II) with a 1,4-bis(prop-2-enyloxy)-9,10-anthraquinone modified carbon paste electrode. Talanta 55, 305–312 (2001)
Tian, M., He, Y.N., Wang, J.L.: Subspeciation of NiS in sulfidic nickel by carbon paste electrode voltammetry. Talanta 55, 349–356 (2001)
Abu-Shawish, H.M.: Potentiometric response of modified carbon paste electrode based on mixed ion-exchangers. Electroanalysis 20, 491–497 (2008)
Gismera, M.J., Sevilla, M.T., Procopio, J.R.: Copper(II) ion-selective electrodes based on dithiosalicylic and thiosalicylic acids. Talanta 74, 190–197 (2007)
Mashhadizadeh, M.H., Eskandari, Kh., Foroumadi, A., Shafiee, A.: Copper(II) modified carbon paste electrodes based on self-assembled mercapto compounds-gold-nanoparticle. Talanta 76, 497–502 (2008)
Mashhadizadeh, M.H., Sheikhshoaie, I.: Mercury(II) ion-selective polymeric membrane sensor based on a recently synthesized Schiff base. Talanta 60, 73–80 (2003)
Shamsipur, M., Mashhadizadeh, M.H.: Cadmium ion-selective electrode based on tetrathia-12-crown-4. Talanta 53, 1065–1071 (2001)
Mashhadizadeh, M.H., Shamsipur, M.: Silver(I)-selective membrane electrode based on hexathia-18-crown-6. Anal. Chim. Acta 381, 111–116 (1999)
Mashhadizadeh, M.H., Mostafavi, A., Allah-Abedi, H., Sheikhshoai, I.: New Schiff base modified carbon paste and coated wire PVC membrane electrode for silver ion. Sens. Actuators B 113, 930–936 (2006)
Mashhadizadeh, M.H., Momeni, A., Razavi, R.: Cobalt(II)-selective membrane electrode using a recently synthesized mercapto compound. Anal. Chim. Acta 462, 245–252 (2002)
Mashhadizadeh, M.H., Sheikhshoaie, I., Saeid-Nia, S.: Nickel(II)-selective membrane potentiometric sensor using a recently synthesized Schiff base as neutral carrier. Sens. Actuators B 94, 241–246 (2003)
Mashhadizadeh, M.H., Sheikhshoaie, I., Monadi, N.: A novel ion selective membrane potentiometric sensor for direct determination of Fe(III) in the presence of Fe(II). Talanta 64, 1048–1052 (2004)
Shockravi, A., Shamsipur, M., Fattahi, H., Taghdiri, M., Heidaryan, D., Alizadeh, K., Rostami, E., Abbaszadeh, M., Yousefi, A.: Efficient synthesis and metal cations complexation of some novel dinaphthosulfide-substituted macrocyclic diamides. J. Incl. Phenom. Macrocycle Chem. 61, 153–160 (2008)
Kamata, S., Bhale, A., Fukunaga, Y., Murata, A.: Copper(II)-selective electrode using thiuram disulfide neutral carriers. Anal. Chem. 60, 2464–2467 (1988)
Umezawa, Y., Umezawa, K., Sato, H.: Selectivity coefficients for ion-selective electrodes: recommended methods for reporting \( K_{A,B}^{pot} \) values. Pure Appl. Chem. 67:507–518 (1995)
Mashhadizadeh, M.H., Pour Taheri, E., Sheikhshoaie, I.: A novel Mn2+ PVC membrane electrode based on a recently synthesized Schiff base. Talanta 72, 1088–1092 (2007)
IUPAC Analytical Chemistry Division: Recommendations for nomenclature of ion selective electrodes. Pure Appl. Chem. 48, 127–132 (1976)
Singh, A.K., Mehtab, S.: Polymeric membrane sensors based on Cd(II) Schiff base complexes for selective iodide determination in environmental and medicinal samples. Talanta 74, 806–814 (2008)
Gholivand, M.B., Rahimi-Nasrabadi, M., Ganjali, M.R., Salavati-Niasari, M.: Highly selective and sensitive copper membrane electrode based on a new synthesized Schiff base. Talanta 73, 553–560 (2007)
Abbaspour, A., Moosavi, S.M.M.: Chemically modified carbon paste electrode for determination of copper(II) by potentiometric method. Talanta 56, 91–96 (2002)
Abu-Shawish, H.M., Saadehb, S.M., Hussien, A.R.: Enhanced sensitivity for Cu(II) by a salicylidine-functionalized polysiloxane carbon paste electrode. Talanta 76, 941–948 (2008)
Javanbakht, M., Badiei, A., Ganjali, M.R., Norouzi, P., Hasheminasab, A., Abdouss, M.: Use of organofunctionalized nanoporous silica gel to improve the lifetime of carbon paste electrode for determination of copper(II) ions. Anal. Chim. Acta 601, 172–182 (2007)
Mashhadizadeh, M.H., Mostafavi, A., Razavi, R., Shamsipur, M.: Highly selective Cu(II) PVC membrane electrode based on 3, 6, 9, 14-tetrathiabicyclo[9.2.1]tetradeca-11, 13-diene as suitable neutral ionophore. Sens. Actuators B 86, 222–228 (2002)
Mittal, S.K., Kumar, A., Gupta, S.K.N., Kaur, S., Kumar, S.: 8-Hydroxyquinoline based neutral tripodal ionophore as a copper (II) selective electrode and the effect of remote substitutents on electrode properties. Anal. Chim. Acta 585, 161–170 (2007)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mashhadizadeh, M.H., Khani, H. & Shockravi, A. Used a new aza-thia-macrocycle as a suitable carrier in potentiometric sensor of copper (II). J Incl Phenom Macrocycl Chem 68, 219–227 (2010). https://doi.org/10.1007/s10847-010-9770-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9770-z