Abstract
Insufficiency of oocyte activation impairs the subsequent embryo development in assisted reproductive technology (ART). Intracellular Ca2+ concentration ([Ca2+]i) oscillations switch the oocytes to resume the second meiosis and initiate embryonic development. However, the [Ca2+]i oscillation patterns in oocytes are poorly characterized. In this study, we investigated the effects of various factors, such as the oocytes age, pH, cumulus cells, in vitro or in vivo maturation, and ER stress on [Ca2+]i oscillation patterns and pronuclear formation after parthenogenetic activation of mouse oocytes. Our results showed that the oocytes released to the oviduct at 17 h post-human chorionic gonadotrophin (hCG) displayed a significantly stronger [Ca2+]i oscillation, including higher frequency, shorter cycle, and higher peak, compared with oocytes collected at earlier or later time points. [Ca2+]i oscillations in acidic conditions (pH 6.4 and 6.6) were significantly weaker than those in neutral and mildly alkaline conditions (pH from 6.8 to 7.6). In vitro-matured oocytes showed reduced frequency and peak of [Ca2+]i oscillations compared with those matured in vivo. In vitro-matured oocytes from the cumulus-oocyte complexes (COCs) showed a significantly higher frequency, shorter cycle, and higher peak compared with the denuded oocytes (DOs). Finally, endoplasmic reticulum stress (ER stress) severely affected the parameters of [Ca2+]i oscillations, including elongated cycles and lower frequency. The pronuclear (PN) rate of oocytes after parthenogenetic activation was correlated with [Ca2+]i oscillation pattern, decreasing with oocyte aging, cumulus removal, acidic pH, and increasing ER stress. These results provide fundamental but critical information for the mechanism of how these factors affect oocyte activation.




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: potential need and demand for infertility medical care. Human reproduction (Oxford, England). 2007;22(6):1506–12. https://doi.org/10.1093/humrep/dem046.
Kamel RM. Management of the infertile couple: an evidence-based protocol. Reprod Biol Endocrinol. 2010;8:21. https://doi.org/10.1186/1477-7827-8-21.
Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013;99(5):1324.e1–31.e1. https://doi.org/10.1016/j.fertnstert.2012.11.037.
Intracytoplasmic sperm injection (ICSI) for non-male factor infertility: a committee opinion. Fertility and sterility. 2012;98(6):1395–9. https://doi.org/10.1016/j.fertnstert.2012.08.026.
Rawe VY, Olmedo SB, Nodar FN, Doncel GD, Acosta AA, Vitullo AD. Cytoskeletal organization defects and abortive activation in human oocytes after IVF and ICSI failure. Molecular human reproduction. 2000;6(6):510–6. https://doi.org/10.1093/molehr/6.6.510.
Neri QV, Lee B, Rosenwaks Z, Machaca K, Palermo GD. Understanding fertilization through intracytoplasmic sperm injection (ICSI). Cell calcium. 2014;55(1):24–37. https://doi.org/10.1016/j.ceca.2013.10.006.
Vanden Meerschaut F, Nikiforaki D, Heindryckx B, De Sutter P. Assisted oocyte activation following ICSI fertilization failure. Reproductive biomedicine online. 2014;28(5):560–71. https://doi.org/10.1016/j.rbmo.2014.01.008.
Ozil JP, Markoulaki S, Toth S, Matson S, Banrezes B, Knott JG, et al. Egg activation events are regulated by the duration of a sustained [Ca2+]cyt signal in the mouse. Developmental biology. 2005;282(1):39–54. https://doi.org/10.1016/j.ydbio.2005.02.035.
Ozil JP. Role of calcium oscillations in mammalian egg activation: experimental approach. Biophysical chemistry. 1998;72(1-2):141–52. https://doi.org/10.1016/s0301-4622(98)00130-6.
Ozil JP, Swann K. Stimulation of repetitive calcium transients in mouse eggs. The Journal of physiology. 1995;483(Pt 2):331–46. https://doi.org/10.1113/jphysiol.1995.sp020589.
Yazawa H, Yanagida K, Sato A. Oocyte activation and Ca(2+) oscillation-inducing abilities of mouse round/elongated spermatids and the developmental capacities of embryos from spermatid injection. Human reproduction (Oxford, England). 2001;16(6):1221–8. https://doi.org/10.1093/humrep/16.6.1221.
Wakai T, Fissore RA. Ca(2+) homeostasis and regulation of ER Ca(2+) in mammalian oocytes/eggs. Cell calcium. 2013;53(1):63–7. https://doi.org/10.1016/j.ceca.2012.11.010.
Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. Journal of cellular physiology. 2006;206(3):565–73. https://doi.org/10.1002/jcp.20471.
Ducibella T, Huneau D, Angelichio E, Xu Z, Schultz RM, Kopf GS, et al. Egg-to-embryo transition is driven by differential responses to Ca(2+) oscillation number. Developmental biology. 2002;250(2):280–91.
Chan C, Martin P, Liptrott NJ, Siccardi M, Almond L, Owen A. Incompatibility of chemical protein synthesis inhibitors with accurate measurement of extended protein degradation rates. Pharmacol Res Perspect. 2017;5(5):e00359. https://doi.org/10.1002/prp2.359.
Kaufman MH. Parthenogenetic activation of oocytes. Cold Spring Harbor protocols. 2018;2018(1). https://doi.org/10.1101/pdb.prot094409.
Sfontouris IA, Nastri CO, Lima ML, Tahmasbpourmarzouni E, Raine-Fenning N, Martins WP. Artificial oocyte activation to improve reproductive outcomes in women with previous fertilization failure: a systematic review and meta-analysis of RCTs. Human reproduction (Oxford, England). 2015;30(8):1831–41. https://doi.org/10.1093/humrep/dev136.
Wang X. The expanding role of mitochondria in apoptosis. Genes & development. 2001;15(22):2922–33.
Chi MM, Manchester JK, Yang VC, Curato AD, Strickler RC, Lowry OH. Contrast in levels of metabolic enzymes in human and mouse ova. Biology of reproduction. 1988;39(2):295–307. https://doi.org/10.1095/biolreprod39.2.295.
Li Y, Guo Y, Tang J, Jiang J, Chen Z. New insights into the roles of CHOP-induced apoptosis in ER stress. Acta biochimica et biophysica Sinica. 2014;46(8):629–40. https://doi.org/10.1093/abbs/gmu048.
Hu H, Tian M, Ding C, Yu S. The C/EBP homologous protein (CHOP) transcription factor functions in endoplasmic reticulum stress-induced apoptosis and microbial infection. Frontiers in immunology. 2018;9:3083. https://doi.org/10.3389/fimmu.2018.03083.
Cheng JM, Li J, Tang JX, Chen SR, Deng SL, Jin C, et al. Elevated intracellular pH appears in aged oocytes and causes oocyte aneuploidy associated with the loss of cohesion in mice. Cell cycle (Georgetown, Tex). 2016;15(18):2454–63. https://doi.org/10.1080/15384101.2016.1201255.
Tanghe S, Van Soom A, Nauwynck H, Coryn M, de Kruif A. Minireview: Functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Molecular reproduction and development. 2002;61(3):414–24. https://doi.org/10.1002/mrd.10102.
Eriani K. Fertilization and development of mice (Mus musculus) embryo in vitro after supplementing the extract of Pandanus conoideus. Nusantara Bioence. 2017;9(2).
Kubiak JZ. Mouse oocytes gradually develop the capacity for activation during the metaphase II arrest. Developmental biology. 1989;136(2):537–45. https://doi.org/10.1016/0012-1606(89)90279-0.
Ma SF, Liu XY, Miao DQ, Han ZB, Zhang X, Miao YL, et al. Parthenogenetic activation of mouse oocytes by strontium chloride: a search for the best conditions. Theriogenology. 2005;64(5):1142–57. https://doi.org/10.1016/j.theriogenology.2005.03.002.
Bomsel-Helmreich O, Huyen LV, Durand-Gasselin I, Salat-Baroux J, Antoine JM. Timing of nuclear maturation and cumulus dissociation in human oocytes stimulated with clomiphene citrate, human menopausal gonadotropin, and human chorionic gonadotropin. Fertil Steril. 1987;48(4):586–95. https://doi.org/10.1016/s0015-0282(16)59469-2.
Vincent C, Cheek TR, Johnson MH. Cell cycle progression of parthenogenetically activated mouse oocytes to interphase is dependent on the level of internal calcium. Journal of Cell Science. 1992;103(2):389.
Miao YL, Williams CJ. Calcium signaling in mammalian egg activation and embryo development: the influence of subcellular localization. Mol Reprod Dev. 2012;79(11):742–56. https://doi.org/10.1002/mrd.22078.
Occhipinti R, Boron WF. Mathematical modeling of acid-base physiology. Progress in biophysics and molecular biology. 2015;117(1):43–58. https://doi.org/10.1016/j.pbiomolbio.2015.01.003.
Barritt JA, Cohen J, Brenner CA. Mitochondrial DNA point mutation in human oocytes is associated with maternal age. Reproductive biomedicine online. 2000;1(3):96–100. https://doi.org/10.1016/s1472-6483(10)61946-3.
Eichenlaub-Ritter U, Wieczorek M, Luke S, Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion. 2011;11(5):783–96. https://doi.org/10.1016/j.mito.2010.08.011.
Bjercke S, Tanbo T, Dale PO, Abyholm T. Comparison between two hCG-to-oocyte aspiration intervals on the outcome of in vitro fertilization. J Assist Reprod Genet. 2000;17(6):319–22. https://doi.org/10.1023/a:1009401027251.
Moriwaki K, Nakagawa T, Nakaya F, Hirohashi N, Chiba K. Arrest at metaphase of meiosis I in starfish oocytes in the ovary is maintained by high CO2 and low O2 concentrations in extracellular fluid. Zoological science. 2013;30(11):975–84. https://doi.org/10.2108/zsj.30.975.
Ata B, Shalom-Paz E, Chian RC, Tan SL. In vitro maturation of oocytes as a strategy for fertility preservation. Clinical obstetrics and gynecology. 2010;53(4):775–86. https://doi.org/10.1097/GRF.0b013e3181f9718f.
Taylor IW, Hodson PJ. Cell cycle regulation by environmental pH. Journal of cellular physiology. 1984;121(3):517–25. https://doi.org/10.1002/jcp.1041210310.
FitzHarris G, Baltz JM. Regulation of intracellular pH during oocyte growth and maturation in mammals. Reproduction. 2009;138(4):619–27. https://doi.org/10.1530/REP-09-0112.
Musa-Aziz R, Occhipinti R, Boron WF. Evidence from simultaneous intracellular- and surface-pH transients that carbonic anhydrase IV enhances CO2 fluxes across Xenopus oocyte plasma membranes. American Journal of Physiology-Cell Physiology. 2014;307(9):C814–C40. https://doi.org/10.1152/ajpcell.00050.2014.
Gilchrist RB, Ritter LJ, Armstrong DT. Oocyte-somatic cell interactions during follicle development in mammals. Animal reproduction science. 2004;82-83:431–46. https://doi.org/10.1016/j.anireprosci.2004.05.017.
Mori T, Amano T, Shimizu H. Roles of gap junctional communication of cumulus cells in cytoplasmic maturation of porcine oocytes cultured in vitro. Biology of reproduction. 2000;62(4):913–9. https://doi.org/10.1095/biolreprod62.4.913.
Nikiforaki D, Vanden Meerschaut F, Qian C, De Croo I, Lu Y, Deroo T, et al. Oocyte cryopreservation and in vitro culture affect calcium signalling during human fertilization. Human reproduction (Oxford, England). 2014;29(1):29–40. https://doi.org/10.1093/humrep/det404.
Wang F, Yuan RY, Li L, Meng TG, Fan LH, Jing Y, et al. Mitochondrial regulation of [Ca2+]i oscillations during cell cycle resumption of the second meiosis of oocyte. Cell Cycle. 2018;17(12):1471–86. https://doi.org/10.1080/15384101.2018.1489179.
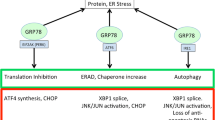
Michalak M, Gye MC. Endoplasmic reticulum stress in periimplantation embryos. Clinical and experimental reproductive medicine. 2015;42(1):1–7. https://doi.org/10.5653/cerm.2015.42.1.1.
Krebs J, Agellon LB, Michalak M. Ca(2+) homeostasis and endoplasmic reticulum (ER) stress: an integrated view of calcium signaling. Biochemical and biophysical research communications. 2015;460(1):114–21. https://doi.org/10.1016/j.bbrc.2015.02.004.
Kaufman RJ, Scheuner D, Schröder M, Shen X, Lee K, Liu CY, et al. The unfolded protein response in nutrient sensing and differentiation. Nature reviews Molecular cell biology. 2002;3(6):411–21. https://doi.org/10.1038/nrm829.
Giorgi C, De Stefani D, Bononi A, Rizzuto R, Pinton P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int J Biochem Cell Biol. 2009;41(10):1817–27. https://doi.org/10.1016/j.biocel.2009.04.010.
Szabadkai G, Bianchi K, Várnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175(6):901–11. https://doi.org/10.1083/jcb.200608073.
Pauly M, Angebault-Prouteau C, Dridi H, Notarnicola C, Scheuermann V, Lacampagne A, et al. ER stress disturbs SR/ER-mitochondria Ca(2+) transfer: Implications in Duchenne muscular dystrophy. Biochimica et biophysica acta Molecular basis of disease. 2017;1863(9):2229–39. https://doi.org/10.1016/j.bbadis.2017.06.009.
Rozpedek W, Pytel D, Mucha B, Leszczynska H, Diehl JA, Majsterek I. The role of the PERK/eIF2α/ATF4/CHOP signaling pathway in tumor progression during endoplasmic reticulum stress. Current molecular medicine. 2016;16(6):533–44. https://doi.org/10.2174/1566524016666160523143937.
Code availability
Not applicable.
Funding
This work was sponsored by the National Natural Science Foundation of China (No 81671425 and No 81901477).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was approved by the Laboratory Animal Care and Use Committee of the Institute of Zoology, Chinese Academy of Sciences.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplemental Figure S1
Intracellular free calcium ([Ca2+]i) oscillations and parameters. A to E indicate different stages of two continuous [Ca2+]i oscillations. “Ca2+ move speed" was the changing fluorescence value every 20 s. We defined the length from the beginning of first [Ca2+]i rise to the beginning of next [Ca2+]i rise as one "cycle"; time from beginning to end of an [Ca2+]i rise as “maintain”; the maximum fluorescence value and maximum changing fluorescence value as “peak” and “move”; and “△peak" was defined as maximum [Ca2+]i fluorescence value minus baseline values. The baseline was defined as the stable minimum [Ca2+]i fluorescence value. The bright field and fluorescent embryo image specifications were as follows: magnification: 200X, time-point: 8008.867s - 9732.370s, wavelength: 191s. The embryos were derived from the 17 h group, and Fluo-4AM fluorescent dye was used. (JPG 160 kb)
Supplemental Figure S2
Relative gene expression was determined using the 2ΔΔCt method normalized to GAPDH. Expression of GRP78 and Chop in oocytes with endoplasmic reticulum stress-induced by tunicamycin (TM) in a dose-dependent manner. RNA was extracted from 100 mouse oocytes for each group. Data represent mean±SD, and Chi-square test and one-way ANOVA are used for statistical analysis. *P<0.05 versus the control group. (JPG 137 kb)
Rights and permissions
About this article
Cite this article
Yuan, RY., Wang, F., Li, S. et al. Maturation conditions, post-ovulatory age, medium pH, and ER stress affect [Ca2+]i oscillation patterns in mouse oocytes. J Assist Reprod Genet 38, 1373–1385 (2021). https://doi.org/10.1007/s10815-021-02100-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02100-9