Abstract
Purpose
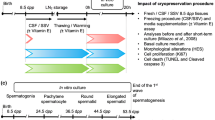
Testicular tissue cryopreservation prior to gonadotoxic therapies is a method to preserve fertility in children. However, the technique still requires development, especially when the tissue is immature and rather susceptible to stress derived from in vitro manipulation. This study aimed to investigate the effects of vitrification with a new cryodevice (E.Vit) on cell membrane integrity and gene expression of prepubertal testicular tissue in the ovine model.
Methods
Pieces of immature testicular tissue (1 mm3) were inserted into “E.Vit” devices and vitrified with a two-step protocol. After warming, tissues were cultured in vitro and cell membrane integrity was assessed after 0, 2, and 24 h by trypan blue exclusion test. Controls consisted of non-vitrified tissue analyzed after 0, 2, and 24 h in vitro culture (IVC). Expression of genes involved in transcriptional stress response (BAX, SOD1, CIRBP, HSP90AB1), cell proliferation (KIF11), and germ- (ZBDB16, TERT, POU5F1, KIT) and somatic- (AR, FSHR, STAR) cell specific markers was evaluated 2 and 24 h after warming.
Results
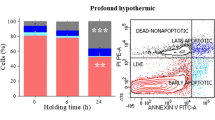
Post-warming trypan blue staining showed the survival of most cells, although membrane integrity immediately after warming (66.00% ± 4.73) or after 2 h IVC (59.67% ± 4.18) was significantly lower than controls (C0h 89.67% ± 1.45). Extended post-warming IVC (24 h) caused an additional decrease to 31% ± 3.46 (P < 0.05). Germ- and somatic-cell specific markers showed the survival of both cell types after cryopreservation and IVC. All genes were affected by cryopreservation and/or IVC, and moderate stress conditions were indicated by transcriptional stress response.
Conclusions
Vitrification with the cryodevice E.Vit is a promising strategy to cryopreserve prepubertal testicular tissue.



Similar content being viewed by others
References
Onofre J, Baert Y, Faes K, Goossens E. Cryopreservation of testicular tissue or testicular cell suspensions: a pivotal step in fertility preservation. Hum Reprod Update. 2016;22:744–61.
Jahnukainen K, Hou M, Petersen C, Setchell B, Söder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–10.
Fattahi A, Latifi Z, Ghasemnejad T, Nejabati HR, Nouri M. Insights into in vitro spermatogenesis in mammals: past, present, future. Mol Reprod Dev. 2017;84:560–75.
Gassei K, Orwig KE. Experimental methods to preserve male fertility and treat male factor infertility. Fertil Steril. 2016;105:256–66.
Ginsberg JP, Li Y, Carlson CA, Gracia CR, Hobbie WL, Miller VA, et al. Testicular tissue cryopreservation in prepubertal male children: an analysis of parental decision-making. Pediatr Blood Cancer. 2014;61:1673–8.
Martinez F, International Society for Fertility Preservation–ESHRE–ASRM Expert Working Group. Update on fertility preservation from the Barcelona International Society for Fertility Preservation-ESHRE-ASRM 2015 expert meeting: indications, results and future perspectives. Fertil Steril. 2017;108:407–415.e11.
Unni S, Kasiviswanathan S, D'Souza S, Khavale S, Mukherjee S, Patwardhan S, et al. Efficient cryopreservation of testicular tissue: effect of age, sample state, and concentration of cryoprotectant. Fertil Steril. 2012;97:200–8.e1.
Wyns C, Curaba M, Petit S, Vanabelle B, Laurent P, Wese JF, et al. Management of fertility preservation in prepubertal patients: 5 years’ experience at the Catholic University of Louvain. Hum Reprod. 2011;26:737–47.
Baert Y, Goossens E, van Saen D, Ning L, in’t Veld P, Tournaye H. Orthotopic grafting of cryopreserved prepubertal testicular tissue: in search of a simple yet effective cryopreservation protocol. Fertil Steril. 2012;97:1152–7.e1–2.
Poels J, Van Langendonckt A, Many MC, Wese FX, Wyns C. Vitrification preserves proliferation capacity in human spermatogonia. Hum Reprod. 2013;28:578–89.
Macente BI, Toniollo GH, Apparicio M, Mansano C, Thomé HE, Canella CL, et al. Evaluation of different fragment sizes and cryoprotectants for cryopreservation of feline testicular tissues. Reprod Domest Anim. 2017;52(Suppl 2):242–7.
Curaba M, Verleysen M, Amorim CA, Dolmans MM, Van Langendonckt A, Hovatta O, et al. Cryopreservation of prepubertal mouse testicular tissue by vitrification. Fertil Steril. 2011;95:229–34.e1.
Zarandi NP, Galdon G, Kogan S, Atala A, Sadri-Ardekani H. Cryostorage of immature and mature human testis tissue to preserve spermatogonial stem cells (SSCs): a systematic review of current experiences toward clinical applications. Stem Cells Cloning. 2018;11:23–38.
Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–7.
Yokonishi T, Sato T, Komeya M, Katagiri K, Kubota Y, Nakabayashi K, et al. Offspring production with sperm grown in vitro from cryopreserved testis tissues. Nat Commun. 2014;5:4320.
Kaneko H, Kikuchi K, Nakai M, Somfai T, Noguchi J, Tanihara F, et al. Generation of live piglets for the first time using sperm retrieved from immature testicular tissue cryopreserved and grafted into nude mice. PLoS One. 2013;8:e70989.
Fayomi AP, Peters K, Sukhwani M, Valli-Pulaski H, Shetty G, Meistrich ML, et al. Autologous grafting of cryopreserved prepubertal rhesus testis produces sperm and offspring. Science. 2019;363:1314–9.
Gholami M, Hemadi M, Saki G, Zendedel A, Khodadadi A, Mohammadi-Asl J. Does prepubertal testicular tissue vitrification influence spermatogonial stem cells (SSCs) viability. J Assist Reprod Genet. 2013;30:1271–7.
Rondanino C, Maouche A, Dumont L, Oblette A, Rives N. Establishment, maintenance and functional integrity of the blood-testis barrier in organotypic cultures of fresh and frozen/thawed prepubertal mouse testes. Mol Hum Reprod. 2017;23:304–20.
Zhang XG, Li H, Hu JH. Effects of various cryoprotectants on the quality of frozen-thawed immature bovine (Qinchuan cattle) calf testicular tissue. Andrologia. 2017;49:9.
Li H, Bian YL, Schreurs N, Zhang XG, Raza SHA, Fang Q, et al. Effects of five cryoprotectants on proliferation and differentiation-related gene expression of frozen-thawed bovine calf testicular tissue. Reprod Domest Anim. 2018;53:1211–8.
Arav A, Natan Y, Kalo D, Komsky-Elbaz A, Roth Z, Levi-Setti PE, et al. A new, simple, automatic vitrification device: preliminary results with murine and bovine oocytes and embryos. J Assist Reprod Genet. 2018;35:1161–8.
Benvenutti L, Salvador RA, Til D, Senn AP, Tames DR, Amaral NLL, et al. Wistar rats immature testicular tissue vitrification and heterotopic grafting. JBRA Assist Reprod. 2018;22:167–73.
Karlsson JO, Toner M. Long-term storage of tissues by cryopreservation: critical issues. Biomaterials. 1996;17:243–56.
Yavin S, Arav A. Measurement of essential physical properties of vitrification solutions. Theriogenology. 2007;67:81–9.
De Gendt K, Verhoeven G. Tissue- and cell-specific functions of the androgen receptor revealed through conditional knockout models in mice. Mol Cell Endocrinol. 2012;352:13–25.
Nishiyama H, Danno S, Kaneko Y, Itoh K, Yokoi H, Fukumoto M, et al. Decreased expression of cold-inducible RNA-binding protein (CIRP) in male germ cells at elevated temperature. Am J Pathol. 1998;152:289–96.
Kliesch S, Penttilä TL, Gromoll J, Saunders PT, Nieschlag E, Parvinen M. FSH receptor mRNA is expressed stage-dependently during rat spermatogenesis. Mol Cell Endocrinol. 1992;84:R45–9.
Gruppi CM, Zakeri ZF, Wolgemuth DJ. Stage and lineage-regulated expression of two hsp90 transcripts during mouse germ cell differentiation and embryogenesis. Mol Reprod Dev. 1991;28:209–17.
Zhang L, Tang J, Haines CJ, Feng HL, Lai L, Teng X, et al. c-kit and its related genes in spermatogonial differentiation. Spermatogenesis. 2011;1:186–94.
Pauls K, Schorle H, Jeske W, Brehm R, Steger K, Wernert N, et al. Spatial expression of germ cell markers during maturation of human fetal male gonads: an immunohistochemical study. Hum Reprod. 2006;21:397–404.
Selvaratnam J, Paul C, Robaire B. Male rat germ cells display age-dependent and cell-specific susceptibility in response to oxidative stress challenges. Biol Reprod. 2015;93:72.
Van Saen D, Pino Sánchez J, Ferster A, van der Werff ten Bosch J, Tournaye H, Goossens E. Is the protein expression window during testicular development affected in patients at risk for stem cell loss? Hum Reprod. 2015;30:2859–70.
Ravindranath N, Dalal R, Solomon B, Djakiew D, Dym M. Loss of telomerase activity during male germ cell differentiation. Endocrinology. 1997;138:4026–9.
Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2001;36:653–9.
Stolz A, Ertych N, Bastians H. A phenotypic screen identifies microtubule plus end assembly regulators that can function in mitotic spindle orientation. Cell Cycle. 2015;14:827–37.
Jiang Y, Xie M, Chen W, Talbot R, Maddox JF, Faraut T, et al. The sheep genome illuminates biology of the rumen and lipid metabolism. Science. 2014;344:1168–73.
Wu H, Yu XL, Guo XF, Zhang F, Pei XZ, Li XX, et al. Effect of liquid helium vitrification on the ultrastructure and related gene expression of mature bovine oocytes after vitrifying at immature stage. Theriogenology. 2017;87:91–9.
Wen Y, Zhao S, Chao L, Yu H, Song C, Shen Y, et al. The protective role of antifreeze protein 3 on the structure and function of mature mouse oocytes in vitrification. Cryobiology. 2014;69:394–401.
Yan W, Suominen J, Samson M, Jegou B, Toppari J. Involvement of Bcl-2 family proteins in germ cell apoptosis during testicular development in the rat and pro-survival effect of stem cell factor on germ cells in vitro. Mol Cell Endocrinol. 2000;165:115–29.
Song YS, Narasimhan P, Kim GS, Jung JE, Park EH, Chan PH. The role of Akt signaling in oxidative stress mediates NF-kappaB activation in mild transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1917–26.
Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol. 1997;137:899–908.
Fujita J. Cold shock response in mammalian cells. J Mol Microbiol Biotechnol. 1999;1:243–55.
Wellmann S, Bührer C, Moderegger E, Zelmer A, Kirschner R, Koehne P, et al. Oxygen-regulated expression of the RNA-binding proteins RBM3 and CIRP by a HIF-1-independent mechanism. J Cell Sci. 2004;117:1785–94.
Liu DH, Yuan HY, Cao CY, Gao ZP, Zhu BY, Huang HL, et al. Heat shock protein 90 acts as a molecular chaperone in late-phase activation of extracellular signal-regulated kinase 1/2 stimulated by oxidative stress in vascular smooth muscle cells. Acta Pharmacol Sin. 2007;28:1907–13.
Wang XH, Kang L. Differences in egg thermotolerance between tropical and temperate populations of the migratory locust Locusta migratoria (Orthoptera: Acridiidae). J Insect Physiol. 2005;51:1277–85.
Haase M, Fitze G. HSP90AB1: helping the good and the bad. Gene. 2016;575(2 Pt 1):171–86.
Meyerson M, Counter CM, Eaton EN, Ellisen LW, Steiner P, Caddle SD, et al. hEST2, the putative human telomerase catalytic subunit gene, is up-regulated in tumor cells and during immortalization. Cell. 1997;90:785–95.
Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43(2 Pt 1):405–13.
Kent D, Copley M, Benz C, Dykstra B, Bowie M, Eaves C. Regulation of hematopoietic stem cells by the steel factor/KIT signaling pathway. Clin Cancer Res. 2008;14:1926–30.
Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–56.
Zaehres H, Lensch MW, Daheron L, Stewart SA, Itskovitz-Eldor J, Daley GQ. High-efficiency RNA interference in human embryonic stem cells. Stem Cells. 2005;23:299–305.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76.
Wang X, Zhao Y, Xiao Z, Chen B, Wei Z, Wang B, et al. Alternative translation of OCT4 by an internal ribosome entry site and its novel function in stress response. Stem Cells. 2009;27:1265–75.
Byun K, Kim TK, Oh J, Bayarsaikhan E, Kim D, Lee MY, et al. Heat shock instructs hESCs to exit from the self-renewal program through negative regulation of OCT4 by SAPK/JNK and HSF1 pathway. Stem Cell Res. 2013;11(3):1323–34.
Miki T, Wong W, Zhou E, Gonzalez A, Garcia I, Grubbs BH. Biological impact of xeno-free chemically defined cryopreservation medium on amniotic epithelial cells. Stem Cell Res Ther. 2016;7:8.
Idda A, Bebbere D, Corona G, Masala L, Casula E, Cincotti A, et al. Insights on cryopreserved sheep fibroblasts by cryomicroscopy and gene expression analysis. Biopreserv Biobank. 2017;15:310–20.
Funding
Research was supported by Regione Autonoma della Sardegna.- L.R. 7-MIGLIOVINGENSAR Project and by Bando Competitivo Fondazione di Sardegna – 2016, CUP J86C18000800005.
DB is the recipient of an RTDa contract at the University of Sassari, Italy, granted by “P.O.R. SARDEGNA F.S.E. 2014–2020” – CUP J86C18000270002.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bebbere, D., Pinna, S., Nieddu, S. et al. Gene expression analysis of ovine prepubertal testicular tissue vitrified with a novel cryodevice (E.Vit). J Assist Reprod Genet 36, 2145–2154 (2019). https://doi.org/10.1007/s10815-019-01559-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01559-x