Abstract
Purpose
The IGF signaling cascade exerts important regulatory functions in human ovarian folliculogenesis. The scope of this study was to evaluate the transcription profile of insulin-like growth factor (IGF) genes during human ovarian follicle development and to analyze follicle fluid levels of key IGF proteins.
Methods
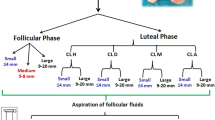
Gene expression profiling was performed with microarray gene analysis. The analysis was assessed from ovarian follicles and granulosa cells (GCs) obtained from isolated stage-specific human ovarian follicles, including preantral follicles, small antral follicles, and preovulatory follicles. Numerous genes involved in the IGF signaling pathway was evaluated and key genes were validated by qPCR from GCs. Protein levels of various IGF components of human follicular fluid (FF) were measured by ELISA and time-resolved immunofluorometric assays (TRIFMA).
Results
The gene expression levels of PAPPA, IGF2, IGF receptors and intracellular IGF-activated genes increased with increasing follicle size. This was especially prominent in the late preovulatory stage where IGF2 expression peaked. Protein levels of intact IGF binding protein-4 decreased significantly in FF from large preovulatory follicles compared with small antral follicles concomitant with higher protein levels of PAPP-A. The IGF modulators IGF-2 receptor, IGFBPs, stanniocalcins, and IGF-2 mRNA binding proteins were all observed to be expressed in the different follicle stages.
Conclusions
This study confirms and highlights the importance of PAPP-A regulating bioactive IGF levels throughout folliculogenesis and especially for the high rate of granulosa cell proliferation and expression of key ovarian hormones important in the last part of the follicular phase of the menstrual cycle.






Similar content being viewed by others
Reference list
Annunziata M, Granata R, Ghigo E. The IGF system. Acta Diabetol. 2011;48;1–9;48:1–9. https://doi.org/10.1007/s00592-010-0227-z.
Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. https://doi.org/10.1016/S0092-8674(05)80085-6.
DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. https://doi.org/10.1038/345078a0.
Le Roith D. The insulin-like growth factor system. Exp Diabesity Res. 2003;4:205–12.
Frasca F, Pandini G, Scalia P, Sciacca L, Mineo R, Costantino A, et al. Insulin receptor isoform a, a newly recognized, high-affinity insulin-like growth factor II receptor in fetal and cancer cells. Mol Cell Biol. 1999;19:3278–88. https://doi.org/10.1128/MCB.19.5.3278.
Mohan S, Baylink DJ, Pettis JL. Insulin-like growth factor (IGF)-binding proteins in serum—do they have additional roles besides modulating the endocrine IGF actions? J Clin Endocrinol Metab. 1996;81:3817–20. https://doi.org/10.1210/jcem.81.11.8923818.
Firth SM, Baxter RC. Cellular actions of the insulin-like growth factor binding proteins. Endocr Rev. 2002;23:824–54. https://doi.org/10.1210/er.2001-0033.
Boldt HB, Conover CA. Pregnancy-associated plasma protein-a (PAPP-A): a local regulator of IGF bioavailability through cleavage of IGFBPs. Growth Hormon IGF Res. 2007;17:10–8. https://doi.org/10.1016/j.ghir.2006.11.003.
Oxvig C. The role of PAPP-A in the IGF system: location, location, location. J Cell Commun Signal. 2015;9:177–87. https://doi.org/10.1007/s12079-015-0259-9.
Laursen LS, Overgaard MT, Söe R, Boldt HB, Sottrup-jensen L, Giudice LC, et al. Pregnancy-associated plasma protein-a (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 2001;504:36–40. https://doi.org/10.1016/S0014-5793(01)02760-0.
Grau S, Richards PJ, Kerr B, Hughes C, Caterson B, Williams AS, et al. The role of human HtrA1 in arthritic disease. J Biol Chem. 2006;281:6124–9. https://doi.org/10.1074/jbc.M500361200.
Mohan S, Thompson GR, Amaar YG, Hathaway G, Tschesche H, Baylink DJ. ADAM-9 is an insulin-like growth factor binding protein-5 protease produced and secreted by human osteoblasts. Biochemistry. 2002;41:15394–403. https://doi.org/10.1021/bi026458q.
Overgaard MT, Boldt HB, Laursen LS, Sottrup-Jensen L, Conover CA, Oxvig C. Pregnancy-associated plasma protein-A2 (PAPP-A2), a novel insulin-like growth factor-binding protein-5 proteinase. J Biol Chem. 2001;276:21849–53. https://doi.org/10.1074/jbc.M102191200.
Laursen LS, Sorensen KK, Andersen MH, Oxvig C. Regulation of insulin-like growth factor bioactivity by sequential proteolytic cleavage of IGF binding protein-4 and -5. Mol Endocrinol. 2007;21:1246–57. https://doi.org/10.1210/me.2006-0522.
Law NC, White MF, Hunzicker-Dunn ME. G protein-coupled receptors (GPCRs) that signal via protein kinase a (PKA) cross-talk at insulin receptor substrate 1 (IRS1) to activate the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. J Biol Chem. 2016;291:27160–9. https://doi.org/10.1074/jbc.M116.763235.
Zha J, Lackner MR. Targeting the insulin-like growth factor receptor-1R pathway for cancer therapy. Clin Cancer Res. 2010;16:2512–7. https://doi.org/10.1158/1078-0432.CCR-09-2232.
Scott CD, Kiess W. Soluble M6P/IGFIIR in the circulation. Best Pract Res Clin Endocrinol Metab. 2015;29:723–33. https://doi.org/10.1016/j.beem.2015.08.001.
Zaina S, Squire S. The soluble type 2 insulin-like growth factor (IGF-II) receptor reduces organ size by IGF-II-mediated and IGF-II-independent mechanisms. J Biol Chem. 1998;273:28610–6. https://doi.org/10.1074/jbc.273.44.28610.
Dessel THJ, Chandrasekher Y, Yap OW, Lee PD, Hintz RL, Faessen GH, et al. Serum and follicular fluid levels of insulin-like growth factor I (IGF-I), IGF-II, and IGF-binding protein-1 and -3 during the normal menstrual cycle. J Clin Endocrinol Metab. 1996;81:1224–31.
Geisthovel F, Moretti-Rojas I, Asch RH, Rojas FJ. Expression of insulin-like growth factor-II (IGF-II) messenger ribonucleic acid (mRNA), but not IGF-I mRNA, in human preovulatory granulosa cells. Hum Reprod. 1989;4:899–902. https://doi.org/10.1093/oxfordjournals.humrep.a137007.
Nyegaard M, Overgaard MT, Su YQ, Hamilton AE, Kwintkiewicz J, Hsieh M, et al. (2010). Lack of functional pregnancy-associated plasma protein-A (PAPP-A) compromises mouse ovarian steroidogenesis and female fertility. Biol Reprod. 2010;82:112–38.
Spicer LJ, Aad PY. Insulin-like growth factor (IGF) 2 stimulates steroidogenesis and mitosis of bovine granulosa cells through the IGF1 receptor: role of follicle-stimulating hormone and IGF2 receptor. Biol Reprod. 2007;77:18–27. https://doi.org/10.1095/biolreprod.106.058230.
Zhou P, Baumgarten SC, Wu Y, Bennett J, Winston N, Hirshfeld-Cytron J, et al. IGF-I signaling is essential for FSH stimulation of AKT and steroidogenic genes in granulosa cells. Mol Endocrinol. 2013;27:511–3. https://doi.org/10.1210/me.2012-1307.
Baumgarten SC, Armouti M, Ko C, Stocco C. IGF1R expression in ovarian granulosa cells is essential for steroidogenesis, follicle survival, and fertility in female mice. Endocrinology. 2017;158:2309–18. https://doi.org/10.1210/en.2017-00146.
Bergh C, Carlsson B, Olsson JH, Selleskog U, Hillensjö T. Regulation of androgen production in cultured human thecal cells by insulin-like growth factor I and insulin. Fertil Steril. 1993;59:323–31. https://doi.org/10.1016/S0015-0282(16)55675-1.
Stubbs SA, Webber LJ, Stark J, Rice S, Margara R, Lavery S, et al. Role of insulin-like growth factors in initiation of follicle growth in normal and polycystic human ovaries. J Clin Endocrinol Metab. 2013;98:3298–305. https://doi.org/10.1210/jc.2013-1378.
Nahum R, Thong KJ, Hillier SG. Metabolic regulation of androgen production by human thecal cells in vitro. Hum Reprod. 1995;10:75–81. https://doi.org/10.1093/humrep/10.1.75.
Spicer LJ, Voge JL, Allen DT. Insulin-like growth factor-II stimulates steroidogenesis in cultured bovine thecal cells. Mol Cell Endocrinol. 2004;227:1–7. https://doi.org/10.1016/j.mce.2004.08.003.
Yuan W, Giudice LC. Insulin-like growth factor-II mediates the steroidogenic and growth promoting actions of follicle stimulating hormone on human ovarian pre-antral follicles cultured in vitro. J Clin Endocrinol Metab. 1999;84:1479–82. https://doi.org/10.1210/jcem.84.4.5727.
Baumgarten SC, Convissar SM, Zamah AM, Fierro MA, Winston NJ, Scoccia B, et al. FSH regulates IGF-2 expression in human granulosa cells in an AKT-dependent manner. J Clin Endocrinol Metab. 2015;100:E1046–55. https://doi.org/10.1210/jc.2015-1504.
Bøtkjær JA, Jeppesen JV, Wissing ML, Kløverpris S, Oxvig C, Mason JI, et al. Pregnancy-associated plasma protein a in human ovarian follicles and its association with intrafollicular hormone levels. Fertil Steril. 2015;104:1294–301. https://doi.org/10.1016/j.fertnstert.2015.07.1152.
Giorgetti C, Vanden Meerschaut F, De Roo C, Saunier O, Quarello E, Hairion D, et al. Multivariate analysis identifies the estradiol level at ovulation triggering as an independent predictor of the first trimester pregnancy-associated plasma protein-A level in IVF/ICSI pregnancies. Hum Reprod. 2013;28:2636–42. https://doi.org/10.1093/humrep/det295.
Hunt LP, McInerney-Leo AM, Sinnott S, Sutton B, Cincotta R, Duncombe G, et al. Low first-trimester PAPP-A in IVF (fresh and frozen-thawed) pregnancies, likely due to a biological cause. J Assist Reprod Genet. 2017;34:1367–75. https://doi.org/10.1007/s10815-017-0996-1.
Dugoff L, Hobbins JC, Malone FD, Porter TF, Luthy D, Comstock CH, et al. First-trimester maternal serum PAPP-A and free-beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population-based screening study (the FASTER trial). Am J Obstet Gynecol. 2004;191:1446–51. https://doi.org/10.1016/j.ajog.2004.06.052.
Kirkegaard I, Henriksen TB, Uldbjerg N. Early fetal growth, PAPP-A and free β-hCG in relation to risk of delivering a small-for-gestational age infant. Ultrasound Obstet Gynecol. 2011;37:341–7. https://doi.org/10.1002/uog.8808.
Jepsen MR, Kløverpris S, Mikkelsen JH, Pedersen JH, Fuchtbauer EM, Laursen LS, et al. Stanniocalcin-2 inhibits mammalian growth by proteolytic inhibition of the insulin-like growth factor axis. J Biol Chem. 2015;290:3430–9. https://doi.org/10.1074/jbc.M114.611665.
Kløverpris S, Mikkelsen JH, Pedersen JH, Jepsen MR, Laursen LS, Petersen SV, et al. Stanniocalcin-1 potently inhibits the proteolytic activity of the metalloproteinase pregnancy-associated plasma protein-A. J Biol Chem. 2015 Sep 4;290:21915–24.
Jepsen MR, Kløverpris S, Bøtkjær JA, Wissing ML, Andersen CY, Oxvig C. The proteolytic activity of pregnancy-associated plasma protein-A is potentially regulated by stanniocalcin-1 and -2 during human ovarian follicle development. Hum Reprod. 2016;31:866–74. https://doi.org/10.1093/humrep/dew013.
Overgaard MT, Haaning J, Boldt HB, Olsen IM, Laursen LS, Christiansen M, et al. Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J Biol Chem. 2000;275:31128–33. https://doi.org/10.1074/jbc.M001384200.
Oxvig C, Sand O, Kristensen T, Gleich GJ, Sottrup-Jensen L. Circulating human pregnancy-associated plasma protein-A is disulfide-bridged to the proform of eosinophil major basic protein. J Biol Chem. 1993;268:12243–6.
Hammer NA, Hansen TV, Byskov AG, Rajpert-De Meyts E, Grøndahl ML, Bredkjaer HE, et al. Expression of IGF-II mRNA-binding proteins (IMPs) in gonads and testicular cancer. Reproduction. 2005;130:203–12. https://doi.org/10.1530/rep.1.00664.
Andersen CY, Kristensen SG, Greve T, Schmidt KT. Cryopreservation of ovarian tissue for fertility preservation in young female oncological patients. Future Oncol. 2012;8:595–608. https://doi.org/10.2217/fon.12.47.
Rosendahl M, Andersen CY, Ernst E, Westergaard LG, Rasmussen PE, Loft A, et al. Ovarian function after removal of an entire ovary for cryopreservation of pieces of cortex prior to gonadotoxic treatment: a follow-up study. Hum Reprod. 2008;23:2475–83. https://doi.org/10.1093/humrep/den248.
Kristensen SG, Rasmussen A, Byskov AG, Andersen CY. Isolation of pre-antral follicles from human ovarian medulla tissue. Hum Reprod. 2011;26:157–66.
Jeppesen JV, Anderson RA, Kelsey TW, Christiansen SL, Kristensen SG, Jayaprakasan K, et al. Which follicles make the most anti-Mullerian hormone in humans? Evidence for an abrupt decline in AMH production at the time of follicle selection. Mol Hum Reprod. 2013;19:519–27. https://doi.org/10.1093/molehr/gat024.
Petersen TS, Kristensen SG, Jeppesen JV, Grøndahl ML, Wissing ML, Macklon KT, et al. Distribution and function of 3′,5′-cyclic-AMP phosphodiesterases in the human ovary. Mol Cell Endocrinol. 2015;403:10–20. https://doi.org/10.1016/j.mce.2015.01.004.
Wissing ML, Kristensen SG, Andersen CY, Mikkelsen AL, Høst T, Borup R, et al. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum Reprod. 2014;29:997–1010. https://doi.org/10.1093/humrep/deu008.
Borgbo T, Povlsen BB, Andersen CY, Borup R, Humaidan P, Grøndahl ML. Comparison of gene expression profiles in granulosa and cumulus cells after ovulation induction with either human chorionic gonadotropin or a gonadotropin-releasing hormone agonist trigger. Fertil Steril. 2013;100:994–1001. https://doi.org/10.1016/j.fertnstert.2013.05.038.
Kristensen SG, Ebbesen P, Andersen CY. Transcriptional profiling of five isolated size-matched stages of human preantral follicles. Mol Cell Endocrinol. 2015;401:189–201. https://doi.org/10.1016/j.mce.2014.12.012.
Bøtkjær JA, Borgbo T, Kløverpris S, Noer PR, Oxvig C, Andersen CY. Effect of pregnancy-associated plasma protein-A (PAPP-A) single-nucleotide polymorphisms on the level and activity of PAPP-A and the hormone profile in fluid from normal human small antral follicles. Fertil Steril. 2016;106:1778–1786.e8. https://doi.org/10.1016/j.fertnstert.2016.09.008.
McCall MN, Bolstad BM, Irizarry RA. Frozen robust multiarray analysis (fRMA). Biostatistics. 2010;11:242–53. https://doi.org/10.1093/biostatistics/kxp059.
McCall MN, Jaffee HA, Irizarry RA. fRMA ST: frozen robust multiarray analysis for Affymetrix exon and gene ST arrays. Bioinformatics. 2012;28:3153–4. https://doi.org/10.1093/bioinformatics/bts588.
Sánchez-Valle J, Tejero H, Ibáñez K, Portero JL, Krallinger M, Al-Shahrour F, et al. A molecular hypothesis to explain direct and inverse co-morbidities between Alzheimer’s disease, glioblastoma and lung cancer. Sci Rep. 2017;7:4474.
Stamatas GN, Wu J, PAPP-As A, Mirmirani P, McCormick TS, Cooper KD, et al. An analysis of gene expression data involving examination of signaling pathways activation reveals new insights into the mechanism of action of minoxidil topical foam in men with androgenetic alopecia. Cell Cycle. 2017;16:1578–84. https://doi.org/10.1080/15384101.2017.1327492.
Chandrasekher YA, Van Dessel HJ, Fauser BC, Giudice LC. Estrogen- but not androgen-dominant human ovarian follicular fluid contains an insulin-like growth factor binding protein-4 protease. J Clin Endocrinol Metab. 1995;80:2734–9. https://doi.org/10.1210/jcem.80.9.7545699.
Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, et al. Pregnancy-associated plasma protein-A is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology. 2001;142:2155. https://doi.org/10.1210/endo.142.5.8286.
Yong EL, Baird DT, Yates R, Reichert LE Jr, Hillier SG. Hormonal regulation of the growth and steroidogenic function of human granulosa cells. J Clin Endocrinol Metab. 1992;74:842–9.
Stocco C, Baumgarten SC, Armouti M, Fierro MA, Winston NJ, Scoccia B, et al. Genome-wide interactions between FSH and insulin-like growth factors in the regulation of human granulosa cell differentiation. Hum Reprod. 2017 Apr 1;32:905–14.
Kristensen SG, Mamsen LS, Jeppesen JV, Bøtkjær JA, Pors SE, Borgbo T, et al. Hallmarks of human small antral follicle development: implications for regulation of ovarian steroidogenesis and selection of the dominant follicle. Front Endocrinol (Lausanne). 2018;8:376.
Baumgarten SC, Convissar SM, Fierro MA, Winston NJ, Scoccia B, Stocco C. IGF1R signaling is necessary for FSH-induced activation of AKT and differentiation of human cumulus granulosa cells. J Clin Endocrinol Metab. 2014;99:2995–3004. https://doi.org/10.1210/jc.2014-1139.
Cataldo NA, Giudice LC. Insulin-like growth factor binding protein profiles in human ovarian follicular fluid correlate with follicular functional status. J Clin Endocrinol Metab. 1992;74:821–9.
El-Roeiy A, Chen X, Roberts VJ, Shimasakai S, Ling N, LeRoith D, et al. Expression of the genes encoding the insulin-like growth factors (IGF-I and II), the IGF and insulin receptors, and IGF-binding proteins-1–6 and the localization of their gene products in normal and polycystic ovary syndrome ovaries. J Clin Endocrinol Metab. 1994;78:1488–96. https://doi.org/10.1210/jcem.78.6.7515389.
Zhou J, Bondy C. Anatomy of the human ovarian insulin-like growth factor system. Biol Reprod. 1993;48:467–82. https://doi.org/10.1095/biolreprod48.3.467.
Voutilainen R, Franks S, Mason HD, Martikainen H. Expression of insulin-like growth factor (IGF), IGF-binding protein, and IGF receptor messenger ribonucleic acids in normal and polycystic ovaries. J Clin Endocrinol Metab. 1996;81:1003–8. https://doi.org/10.1210/jcem.81.3.8772565.
Wu XK, Sallinen K, Anttila L, Mäkinen M, Luo C, Pöllänen P, et al. Expression of insulin-receptor substrate-1 and -2 in ovaries from women with insulin resistance and from controls. Fertil Steril. 2000;74:564–72. https://doi.org/10.1016/S0015-0282(00)00688-9.
Schneider A, Zhi X, Moreira F, Lucia T, Mondadori RG, Masternak MM. Primordial follicle activation in the ovary of Ames dwarf mice. J Ovarian Res. 2014;7:120.
Deol HK, Varghese R, Wagner GF, Dimattia GE. Dynamic regulation of mouse ovarian stanniocalcin expression during gestation and lactation. Endocrinology. 2000;141:3412–21. https://doi.org/10.1210/endo.141.9.7658.
Loddo M, Andryszkiewicz J, Acebes SR, Stoeber K, Jones A, Dafou D, et al. Pregnancy-associated plasma protein a regulates mitosis and is epigenetically silenced in breast cancer. J Pathol. 2014;233:344–56. https://doi.org/10.1002/path.4393.
Mathur SP, Mathur RS, Young RC. Cervical epidermal growth factor-receptor (EGF-R) and serum insulin-like growth factor II (IGF-II) levels are potential markers for cervical cancer. Am J Reprod Immunol. 2000;44:222–30. https://doi.org/10.1111/j.8755-8920.2000.440406.x.
Acknowledgements
We acknowledge the Core Facility for helping with fine microarray analysis. Furthermore, we are thankful for the work performed at the fertility clinics in regard to collecting the granulosa cells and follicular fluids from IVF patients. Finally, we thank Pernille Rimmer Noer from the Department of Molecular Biology and Genetics at University of Aarhus for technical assistance.
Funding
The financial support from The Novo Nordisk Foundation, the Lundbeck Foundation, and Gangstedfonden is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
Additional gene expression profiles of IGF genes during human folliculogenesis (TIF 1.83 MB)
Rights and permissions
About this article
Cite this article
Bøtkjær, J.A., Pors, S.E., Petersen, T.S. et al. Transcription profile of the insulin-like growth factor signaling pathway during human ovarian follicular development. J Assist Reprod Genet 36, 889–903 (2019). https://doi.org/10.1007/s10815-019-01432-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-019-01432-x