Abstract
Purpose
To understand if repeated cycles (2–4 rounds) of gonadotropin stimulation could affect intracellular localization/content of proteins controlling cell cycle progression in mouse fallopian tubes (FT) and ovaries.
Methods
FT and ovaries of estrous mice (control) and of stimulated mice were analyzed to detect Oct-3/4, Sox-2, p53, β-catenin, pAKT and cyclin D1 localization/content. Spindles and chromosome alignment were analyzed in ovulated oocytes.
Results
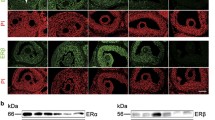
After round 4, FT and ovaries of control and stimulated groups showed no differences in Oct-3/4, Sox-2 and β-catenin localization nor in Oct-3/4, Sox-2, p53, β-catenin and pAKT contents. Cyclin D1 level increased significantly in FT of treated mice. Oocytes number decreased meanwhile frequency of abnormal meiotic spindles increased with treatments.
Conclusions
Repetitive stimulations affected oocyte spindle morphology but did not induce changes in a set of proteins involved in cell cycle progression, usually altered in ovarian cancer. The significant increase of cyclin D1 in the FT requires further investigation.





Similar content being viewed by others
References
Lerner-Geva L, Rabonovici J, Lunenfeld B. Ovarian stimulation: is there a long-term risk for ovarian, breast and endometrial cancer? Womens Health. 2010;6:831–9.
Van Blerkom J, Davis P. Differential effects of repeated ovarian stimulation on cytoplasmic and spindle organization in metaphase II mouse oocytes matured in vivo and in vitro. Hum Reprod. 2001;16:757–64.
Luk J, Arici A. Does the ovarian reserve decrease from repeated ovulation stimulations? Curr Opin Obstet Gynecol. 2010;22:177–82.
Gadducci A, Guerrieri ME, Genazzani AR. Fertility drug use and risk of ovarian tumors: a debated clinical challenge. Gynecol Endocrinol. 2013;29:30–5.
Sanner K, Conner P, Bergfeldt K, Dickman P, Sundfeldt K, Bergh T, et al. Ovarian epithelial neoplasia after hormonal infertility treatment: long-term follow-up of a historical cohort in Sweden. Fertil Steril. 2009;91:1152–8.
Källén B, Finnström O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Malignancies among women who gave birth after in vitro fertilization. Hum Reprod. 2011;26:253–8.
Vlahos NF, Economopoulos KP, Creatsas G. Fertility drugs and ovarian cancer risk: a critical review of the literature. Ann N Y Acad Sci. 2010;1205:214–9.
Britt K, Short R. The plight of nuns: hazards of nulliparity. Lancet. 2012;379:2322–3.
Jensen A, Sharif H, Frederiksen K, Kjaer SK. Use of fertility drugs and risk of ovarian cancer: Danish population based cohort study. BMJ. 2009;338:b249.
van Leeuwen FE, Klip H, Mooji TM, van de Swaluw AM, Lambalk CB, Kortman M, et al. Risk of borderline and invasive ovarian tumours after ovarian stimulation for in vitro fertilization in a large Dutch cohort. Hum Reprod. 2011;26:3456–65.
Twombly R. Too early to determine cancer risk from infertility treatments. J Natl Cancer Inst. 2012;104:501–2.
American Cancer Society. Cancer facts & figures 2013. Atlanta: American Cancer Society; 2013.
Romero I, Bast Jr RC. Minireview: human ovarian cancer: biology, current management, and paths to personaliziong therapy. Endocrinology. 2012;153:1593–602.
Fathalla MF. Incessant ovulation – a factor in ovarian neoplasia? Lancet. 1971;2:163.
Vanderhyden BC. Loss of ovarian function and the risk of ovarian cancer. Cell Tissue Res. 2005;322:117–24.
Kurman RJ, Shih IM. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43.
Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci U S A. 2012;109:3921–6.
Cibula D, Widschwendter M, Màjek O, Dusek L. Tubal ligation and the risk of ovarian cancer: review and meta-analysis. Hum Reprod Update. 2011;17:55–67.
Marquez RT, Baggerly KA, Patterson AP, et al. Patterns of gene expression in different histotypes of epithelial ovarian cancer correlate with those in normal fallopian tube, endometrium, and colon. Clin Cancer Res. 2005;11:6116–26.
Zhang J, Li YL, Zhou CY, Hu YT, Chen HZ. Expression of octamer-4 in serous and mucinous ovarian carcinoma. J Clin Pathol. 2010;63:879–83.
Ye F, Li Y, Hu Y, Zhou C, Hu Y, Chen H. Expression of Sox2 in human ovarian epithelial carcinoma. J Cancer Res Clin Oncol. 2011;137:131–7.
Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nat Genet. 2000;24:372–6.
Liu K, Lin B, Zhao M, Yang X, Chen M, Gao A, et al. The multiple roles for Sox2 in stem cell maintenance and tumorigenesis. Cell Signal. 2013;25:1264–71.
Sung MT, Jones TD, Beck SD, Foster RS, Cheng L. OCT4 is superior to CD30 in the diagnosis of metastatic embryonal carcinomas after chemotherapy. Hum Pathol. 2006;37:662–7.
Santagata S, Ligon KL, Hornick JL. Embryonic stem cell transcription factor signatures in the diagnosis of primary and metastatic germ cell tumors. Am J Surg Pathol. 2007;31:836–45.
Rossi G, Macchiarelli G, Palmerini MG, Canipari R, Cecconi S. Meiotic spindle configuration is differentially influenced by FSH and epidermal growth factor during in vitro maturation of mouse oocytes. Hum Reprod. 2006;21:1765–70.
Combelles CMH, Albertini DF. Assessment of oocyte quality following repeated gonadotropin stimulation in the mouse. Biol Reprod. 2003;68:812–21.
Matoba R, Niwa H, Masui S, Ohtsuka S, CArter MG, Sharov AA, et al. Dissecting Oct3/4-regulated gene networks in embryonic stem cells by expression profiling. PLoS One. 2006;20:1–e26.
Jeong CH, Cho YY, Kim MO, et al. Phosphorylation of Sox2 cooperates in reprogramming to pluripotent stem cells. Stem Cells. 2010;28:2141–50.
Masui S, Nakatabe Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K, et al. Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat Cell Biol. 2007;9:625–35.
Jia X, Li X, Xu Y, Zhang S, Mou W, Liu Y, et al. SOX2 promotes tumorigenesis and increases the anti-apoptotic property of human prostate cancer cell. J Mol Cell Biol. 2011;3:230–8.
Papapetrou EP, Tomishima MJ, Chambers SM, Mica Y, Reed E, Menon J, et al. Stoichiometric and temporal requirements of Oct4, Sox2, Klf4, and c-Myc expression for efficient human iPSC induction and differentiation. Proc Natl Acad Sci U S A. 2009;106:12759–64.
Gao Z, Cox JL, Gilmore JM, Ormsbee BD, Mallann SK, Washburn MP, et al. Determination of protein interactome of transcription factor Sox2 in embryonic stem cells engineered for inducible expression of four reprogramming factors. J Biol Chem. 2012;287:11384–97.
Cecconi S, Mauro A, Cellini V, Patacchiola F. The role of Akt signalling in the mammalian ovary. Int J Dev Biol. 2012;56:809–17.
Saifo MS, Rempinski Jr DR, Rustum YM, Azrak RG. Targeting the oncogenic protein beta-catenin to enhance chemotherapy outcome against solid human cancers. Mol Cancer. 2010;9:310.
Mazzoletti M, Broggini M. PI3K/AKT/mTOR inhibitors in ovarian cancer. Curr Med Chem. 2010;17:4433–47.
Li W, Cai JH, Zhang J, Tang YX, Wan L. Effects of cyclooxygenase inhibitors in combination with taxol on expression of cyclin d1 and ki-67 in a xenograft model of ovarian carcinoma. Int J Mol Sci. 2012;13:9741–53.
Lalwani N, Shanbhogue AK, Vikram R, Nagar A, Jagirdar J, Prasad SR. Current update on borderline ovarian neoplasms. AJR Am J Roentgenol. 2010;194:330–6.
Acknowledgments
This work has been funded by the Italian Ministry of Education, University and Research to S.C.and G.C. (ex 60 %), and by FARI 2012, “Sapienza” University of Rome to R.C.
The study has been performed in the framework of the “Research Centre for Molecular Diagnostics and Advanced Therapies”. The authors wish to thank the “Abruzzo earthquake relief fund” (Toronto, Ontario) that supported in part this research with the purchase of confocal microscope Leica TCS SP5 II (Leica, Germany).
S.C. dedicates this paper in the memory of Daniela Lombardi (1956–2012).
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
In mouse ovary and fallopian tubes, four rounds of gonadotropin stimulation did not modify cell cycle proteins contents, usually altered in ovarian cancer.
Rights and permissions
About this article
Cite this article
Di Luigi, G., Rossi, G., Castellucci, A. et al. Repeated ovarian stimulation does not affect the expression level of proteins involved in cell cycle control in mouse ovaries and fallopian tubes. J Assist Reprod Genet 31, 717–724 (2014). https://doi.org/10.1007/s10815-014-0198-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-014-0198-z