Abstract
Purpose
During laboratory manipulations, oocytes and embryos are inevitably exposed to suboptimal conditions that interfere with the normal development of embryos.
Materials and methods
In this study, we examined the effects of antioxidants, feeder cells and a conditioned medium on embryo development and cleavage rate following exposure of the embryos to suboptimal conditions. We exposed mouse two-cell embryos to visible light and divided them into four groups: control (E-ctr), co-culture (Co-c), conditioned medium (Cndm) and antioxidant-plus medium (Aopm). We used human umbilical cord matrix-derived mesenchymal cells for co-culture. A group of embryos was not exposed to visible light and served as the non-exposed control (NE-ctr) group.
Results
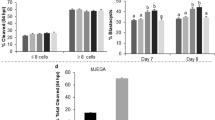
The developmental rate was higher in NE-ctr embryos than in the E-ctr group. Exposed embryos in the various groups showed a comparable developmental rate at different stages. Blastomere number significantly increased (P < 0.05) in the Co-c and Aopm groups compared with the E-ctr and Cndm groups. No significant difference was observed between the Co-c and Aopm groups.
Conclusions
Our data indicate that in suboptimal conditions, antioxidants could improve the embryo cleavage rate in the same way as feeder cells. Antioxidants probably improve embryo quality through their ability to scavenge reactive oxygen species.



Similar content being viewed by others
References
Nematollahi-mahani SN, Pahang H, Moshkdanian G, Nematollahi-mahani A. Effect of embryonic fibroblast cell co-culture on development of mouse embryos following exposure to visible light. J Assist Reprod Genet. 2009;26(2–3):129–35.
Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci USA. 2007;104(36):14289–93.
Elkind MM. Sedimentation of DNA released from Chinese hamster cells. Biophys J. 1971;11(6):502–20.
Lavi R, Shainberg A, Friedmann H, Shneyvays V, Rickover O, Eichler M, et al. Low energy visible light induces reactive oxygen species generation and stimulates an increase of intracellular calcium concentration in cardiac cells. J Biol Chem. 2003;278(42):40917–22.
Guerin P, El Mouatassim S, Menezo Y. Oxidative stress and protection against reactive oxygen species in the pre-implantation embryo and its surroundings. Hum Reprod Update. 2001;7(2):175–89.
Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C, Hardiman P. Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod. 2003;18(11):2270–4.
McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speake BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil. 2000;118(1):163–70.
Sturmey RG, Leese HJ. Energy metabolism in pig oocytes and early embryos. Reproduction. 2003;126(2):197–204.
Gardiner CS, Reed DJ. Glutathione redox cycle-driven recovery of reduced glutathione after oxidation by tertiary-butyl hydroperoxide in preimplantation mouse embryos. Arch Biochem Biophys. 1995;321(1):6–12.
Lapointe S, Sullivan R, Sirard MA. Binding of a bovine oviductal fluid catalase to mammalian spermatozoa. Biol Reprod. 1998;58(3):747–53.
Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–43.
Rose RC, Bode AM. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7(12):1135–42.
Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, Liu Y, Fang FG, Ding JP, and Zhang XR. Effects of L-ascorbic acid, alpha-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim. 45(1):19–25.
Otoi T, Koyama N, Yamamoto K, Horikita N, Tachikawa S, Suzuki T. Developmental competence of frozen-thawed blastocysts from fair-quality bovine embryos cultured with beta-mercaptoethanol. Vet J. 2000;159(3):282–6.
Caamano JN, Ryoo ZY, Thomas JA, Youngs CR. beta-mercaptoethanol enhances blastocyst formation rate of bovine in vitro-matured/in vitro-fertilized embryos. Biol Reprod. 1996;55(5):1179–84.
Hosseini SM, Forouzanfar M, Hajian M, Asgari V, Abedi P, Hosseini L, et al. Antioxidant supplementation of culture medium during embryo development and/or after vitrification-warming; which is the most important? J Assist Reprod Genet. 2009;26(6):355–64.
El Mouatassim S, Guerin P, Menezo Y. Mammalian oviduct and protection against free oxygen radicals: expression of genes encoding antioxidant enzymes in human and mouse. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):1–6.
Nematollahi-Mahani SN, Nematollahi-Mahani A, Moshkdanian G, Shahidzadehyazdi Z, Labibi F. The role of co-culture systems on developmental competence of preimplantation mouse embryos against pH fluctuations. J Assist Reprod Genet. 2009;26:597–604.
Aghaee-afshar M, Rezazadehkermani M, Asadi A, Malekpour-afshar R, Shahesmaeili A, Nematollahi-mahani SN. Potential of human umbilical cord matrix and rabbit bone marrow— derived mesenchymal stem cells in repair of surgically incised rabbit external Anal sphincter. Dis Colon Rectum. 2009;52(10):1753–61.
Thouas GA, Korfiatis NA, French AJ, Jones GM, Trounson AO. Simplified technique for differential staining of inner cell mass and trophectoderm cells of mouse and bovine blastocysts. Reprod Biomed Online. 2001;3(1):25–9.
Lane M, Gardner DK. Selection of viable mouse blastocysts prior to transfer using a metabolic criterion. Hum Reprod. 1996;11(9):1975–8.
Cheng TC, Huang CC, Huang LS, Chen CI, Lee MS, Liu JY. Evaluation of mouse blastocyst implantation rate by morphology grading. Chin J Physiol. 2004;47(1):43–7.
Lutsenko EA, Carcamo JM, Golde DW. Vitamin C prevents DNA mutation induced by oxidative stress. J Biol Chem. 2002;277(19):16895–9.
Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–63.
Schneider M, Diemer K, Engelhart K, Zankl H, Trommer WE, Biesalski HK. Protective effects of vitamins C and E on the number of micronuclei in lymphocytes in smokers and their role in ascorbate free radical formation in plasma. Free Radic Res. 2001;34(3):209–19.
Mozdarani H, Nazari E. Cytogenetic damage in preimplantation mouse embryos generated after paternal and parental gamma-irradiation and the influence of vitamin C. Reproduction. 2009;137(1):35–43.
Lane M, Maybach JM, Gardner DK. Addition of ascorbate during cryopreservation stimulates subsequent embryo development. Hum Reprod. 2002;17(10):2686–93.
Tatemoto H, Ootaki K, Shigeta K, Muto N. Enhancement of developmental competence after in vitro fertilization of porcine oocytes by treatment with ascorbic acid 2-O-alpha-glucoside during in vitro maturation. Biol Reprod. 2001;65(6):1800–6.
Hossein MS, Hashem MA, Jeong YW, Lee MS, Kim S, Kim JH, et al. Temporal effects of alpha-tocopherol and L-ascorbic acid on in vitro fertilized porcine embryo development. Anim Reprod Sci. 2007;100(1–2):107–17.
Wang X, Falcone T, Attaran M, Goldberg JM, Agarwal A, Sharma RK. Vitamin C and vitamin E supplementation reduce oxidative stress-induced embryo toxicity and improve the blastocyst development rate. Fertil Steril. 2002;78(6):1272–7.
Nematollahi-mahani SN, Rezazadeh-kermani M, Latifpour M, Salehinejad P. Biological and biochemical characteristics of human umbilical cord mesenchymal cells. J Reprod Infertil. 2009;10(1):8. abstract in English.
Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, West AB. Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol. 1999;277(1 Pt 1):C1–9.
Tsai FC, Gardner DK. Nicotinamide, a component of complex culture media, inhibits mouse embryo development in vitro and reduces subsequent developmental potential after transfer. Fertil Steril. 1994;61(2):376–82.
Kattal N, Cohen J, Barmat LI. Role of coculture in human in vitro fertilization: a meta-analysis. Fertil Steril. 2008;90(4):1069–76.
Zhang S, Lu C, Lin G, Gong F, Lu G. The number of blastomeres in post-thawing embryos affects the rates of pregnancy and delivery in freeze-embryo-transfer cycles. J Assist Reprod Genet. 2009;26(11–12):569–73.
Acknowledgments
The authors thank members of the Parasitology Department at the Afzalipour School of Medicine, Kerman University of Medical Sciences and the following people: P. Salehinejad from the School of Nursing for her laboratory assistance and F. Moshkdanian for help in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule Antioxidants improved the cleavage rate of mouse embryos that were exposed to suboptimal conditions, at a comparable rate to the co-culture system.
Rights and permissions
About this article
Cite this article
Moshkdanian, G., Nematollahi-mahani, S.N., Pouya, F. et al. Antioxidants rescue stressed embryos at a rate comparable with co-culturing of embryos with human umbilical cord mesenchymal cells. J Assist Reprod Genet 28, 343–349 (2011). https://doi.org/10.1007/s10815-010-9529-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-010-9529-x