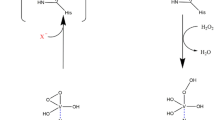
Abstract
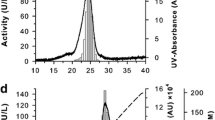
The gene encoding vanadium-dependent bromoperoxidase (V-BPO) was cloned for the first time from the red alga Laurencia saitoi, which produces pharmaceutically promising brominated diterpenoids and triterpenoids. The molecular weight of V-BPO from L. saitoi (LsVBPO1) was the highest (77.0 kDa) among previously reported V-BPOs from Laurencia with a peptide insertion Asn194-Ser221 containing short Gln repeats. It shares approximately 60% amino acid sequence identity with V-BPOs from L. nipponica (LnVBPO1 and LnVBPO2) and L. okamurae (LoVBPO1a and LoVBPO2a). Heterologously expressed LsVBPO1 in Escherichia coli was partially purified and exhibited low but significant bromination activity of 38 U mg−1 protein using monochlorodimedone. The pH optimum was 8.0, which was more alkaline than that for LnVBPOs and LoVBPO2a (pH 7.0). The Km for H2O2 was 0.04 mM, comparable to LnVBPO1 (0.026 mM), LnVBPO2 (0.025 mM), and LoVBPO2a (0.014 mM). LsVPBO1 retained its bromination activity until 45 °C for 20 min. When incubated at 55 °C for 20 min, catalytic activity decreased rapidly, as shown for LnVBPO1 and LoVBPO2a (retained at 45 °C, decreased at 55 °C) and LnVBPO2 (retained at 55 °C, decreased at 65 °C). Unlike other V-BPOs from Laurencia (LnVBPO1, LnVBPO2, and LoVBPO2a), dialysis and concentration during purification process were rapidly inactivated LsVBPO1, suggesting its structural instability.





Similar content being viewed by others
Data availability
Raw data were generated at Hokkaido University. Data supporting the findings of this study are available from the corresponding authors K.K. and T.O. on request.
References
Agarwal V, Miles ZD, Winter JM, Eustáquio AS, El Gamal AA, Moore BS (2017) Enzymatic halogenation and dehalogenation reactions: pervasive and mechanistically diverse. Chem Rev 117:5619–5674
Baharum H, Chu WC, Teo SS, Ng KY, Abdul Rahim R, Ho CL (2013) Molecular cloning, homology modeling and site-directed mutagenesis of vanadium-dependent bromoperoxidase (GcVBPO1) from Gracilaria changii (Rhodophyta). Phytochemistry 92:49–59
Bernhardt P, Okino T, Winter JM, Miyanaga A, Moore BS (2011) A stereoselective vanadium-dependent chloroperoxidase in bacterial antibiotic biosynthesis. J Am Chem Soc 133:4268–4270
Bonney KJ, Braddock DC (2012) A unifying stereochemical analysis for the formation of halogenated C15-acetogenin medium-ring ethers from Laurencia species via intramolecular bromonium ion assisted epoxide ring-opening and experimental corroboration with a model epoxide. J Org Chem 77:9574–9584
Bryan AC, Zhang J, Guo J, Ranjan P, Singan V, Barry K, Schmutz J, Weighill D, Jacobson D, Jawdy S, Tuskan GA, Chen J, Muchero W (2018) A variable polyglutamine repeat affects subcellular localization and regulatory activity of a Populus ANGUSTIFOLIA protein. G3-Genes Genom Genet 8:2631–2641
Butler A, Sandy M (2009) Mechanistic considerations of halogenating enzymes. Nature 460:848–854
Carter JN, Beatty KE, Simpson MT, Butler A (2002) Reactivity of recombinant and mutant vanadium bromoperoxidase from the red alga Corallina officinalis. J Inorg Biochem 91:59–69
Carter-Franklin JN, Butler A (2004) Vanadium bromoperoxidase-catalyzed biosynthesis of halogenated marine natural products. J Am Chem Soc 126:15060–15066
Chan HSS, Nguyen QNN, Paton RS, Burton JW (2019) Synthesis, characterization, and reactivity of complex tricyclic oxonium ions, proposed intermediates in natural product biosynthesis. J Am Chem Soc 141:15951–15962
Collén J, Porcel B, Carré W, Ball SG, Chaparro C, Tonon T, Barbeyron T, Michel G, Noel B, Valentin K et al (2013) Genome structure and metabolic features in the red seaweed Chondrus crispus shed light on evolution of the Archaeplastida. Proc Natl Acad Sci 110:5247–5252
Das A, Weise C, Polack M, Urban RD, Krafft B, Hasan S, Westphal H, Warias R, Schmidt S, Gulder T, Belder D (2022) On-the-fly mass spectrometry in digital microfluidics enabled by a microspray hole: toward multidimensional reaction monitoring in automated synthesis platforms. J Am Chem Soc 144:10353–10360
Fernández JJ, Souto ML, Norte M (2000) Marine polyether triterpenes. Nat Prod Rep 17:235–246
Frank A, Seel CJ, Groll M, Gulder T (2016) Characterization of a cyanobacterial haloperoxidase and evaluation of its biocatalytic halogenation potential. ChemBioChem 17:2028–2032
Fujino Y, Nagai Y (2022) The molecular pathogenesis of repeat expansion diseases. Biochem Soc Trans 50:119–134
Fukuzawa A, Aye M, Takasugi Y, Nakamura M, Tamura M, Murai A (1994) Enzymatic bromo-ether cyclization of laurediols with bromoperoxidase. Chem Lett 23:2307–2310
Hall TA (1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser 41:95–98
Harizani M, Ioannou E, Roussis V (2016) The Laurencia paradox: an endless source of chemodiversity. Prog Chem Org Nat Prod 102:91–252
Hashimoto M, Kan T, Nozaki K, Yanagiya M, Shirahama H, Matsumoto T (1990) Total syntheses of (+)-thyrsiferol, (+)-thyrsiferyl 23-acetate, and (+)-venustatriol. J Org Chem 55:5088–5107
Höfler GT, But A, Hollmann F (2019) Haloperoxidases as catalysts in organic synthesis. Org Biomol Chem 17:9267–9274
Hoshino A, Nakai H, Morino M, Nishikawa K, Kodama T, Nishikibe K, Morimoto Y (2017) Total synthesis of the cytotoxic marine triterpenoid isodehydrothyrsiferol reveals partial enantiodivergency in the thyrsiferol family of natural products. Angew Chem Int Ed 56:3064–3068
Ishikawa T, Washio K, Kaneko K, Tang XR, Morikawa M, Okino T (2022) Characterization of vanadium-dependent bromoperoxidases involved in the production of brominated sesquiterpenes by the red alga Laurencia okamurae. Appl Phycol 3:120–131
Isupov MN, Dalby AR, Brindley AA, Izumi Y, Tanabe T, Murshudov GN, Littlechild JA (2000) Crystal structure of dodecameric vanadium-dependent bromoperoxidase from the red algae Corallina officinalis. J Mol Biol 299:1035–1049
Itoh N, Izumi Y, Yamada H (1986) Characterization of nonheme type bromoperoxidase in Corallina pilulifera. J Biol Chem 261:5194–5200
Kaneko K, Washio K, Umezawa T, Matsuda F, Morikawa M, Okino T (2014) cDNA cloning and characterization of vanadium-dependent bromoperoxidases from the red alga Laurencia nipponica. Biosci Biotechnol Biochem 78:1310–1319
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol 35:1547–1549
Kurata K, Taniguchi K, Agatsuma Y, Suzuki M (1998) Diterpenoid feeding-detergents from Laurencia saitoi. Phymchemistry 47:363–369
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Latham J, Brandenburger E, Shepherd SA, Menon BRK, Micklefield J (2018) Development of halogenase enzymes for use in synthesis. Chem Rev 118:232–269
Littlechild J, Garcia-Rodriguez E (2003) Structural studies on the dodecameric vanadium bromoperoxidase from Corallina species. Coord Chem Rev 237:65–76
Llamas E, Koyuncu S, Lee HJ, Gutierrez-Garcia R, Dunken N, Charura N, Torres-Montilla S, Schlimgen E, Pulido P, Rodriguez-Concepcion M, Zuccaro A, Vilchez D (2022) Chloroplast protein import determines plant proteostasis and retrograde signaling. bioRxiv preprint
McLauchlan CC, Murakami HA, Wallace CA, Crans DC (2019) Coordination environment changes of the vanadium in vanadium-dependent haloperoxidase enzymes. J Inorg Biochem 186:267–279
Mata L, Gaspar H, Justino F, Santos R (2011) Effects of hydrogen peroxide on the content of major volatile halogenated compounds in the red alga Asparagopsis taxiformis (Bonnemaisoniaceae). J Appl Phycol 23:827–832
Messerschmidt A, Wever R (1996) X-ray structure of a vanadium-containing enzyme: Chloroperoxidase from the fungus Curvularia inaequalis. Proc Natl Acad Sci 93:392–396
Ohshiro T, Hemrika W, Aibara T, Wever R, Izumi Y (2002) Expression of the vanadium-dependent bromoperoxidase gene from a marine macro-alga Corallina pilulifera in Saccharomyces cerevisiae and characterization of the recombinant enzyme. Phytochemistry 60:595–601
Ohshiro T, Littlechild J, Garcia-Rodriguez E, Isupov MN, Iida Y, Kobayashi T, Izumi Y (2004) Modification of halogen specificity of a vanadium-dependent bromoperoxidase. Protein Sci 13:1566–1571
Rush C, Willetts A, Davies G, Dauter Z, Watson H, Littlechild J (1995) Purification, crystallisation and preliminary X-ray analysis of the vanadium-dependent haloperoxidase from Corallina officinalis. FEBS Lett 359:244–246
Sheffield DJ, Harry T, Smith AJ, Rogers LT (1993) Purification and characterization of the vanadium bromoperoxidase from the macroalga Corallina officinalis. Phytochemistry 32:21–26
Soedjak HS, Butler A (1990) Chlorination catalyzed by vanadium bromoperoxidase. Inorg Chem 29:5015–5017
Suzuki T, Suzuki M, Furusaki A, Matsumoto T, Kato A, Imanaka Y, Kurosawa E (1985) Teurilene and thyrsiferyl 23-acetate, meso and remarkably cytotoxic compounds from the marine red alga Laurencia obtusa (Hudson) Lamouroux. Tet Lett 26:1329–1332
Suzuki M, Matsuo Y, Takeda S, Suzuki T (1993) Intricatetraol, a halogenated triterpene alcohol from the red alga Laurencia intricata. Phytochemistry 33:651–656
Suzuki M, Vairappan CS (2005) Halogenated secondary metabolites from Japanese species of the red algal genus Laurencia (Rhodomelaceae, Ceramiales). Curr Top Phytochem 7:1–34
Taylor MT, Fox JM (2015) Biosynthesis of the C15-acetogenin laurepoxide may involve bromine-induced skeletal rearrangement of a Δ4-oxocene precursor. Tet Lett 56:3560–3563
Undurraga SF, Press MO, Legendre M, Bujdoso N, Bale J, Wang H, Davis SJ, Verstrepen KJ, Queitsch C (2012) Background-dependent effects of polyglutamine variation in the Arabidopsis thaliana gene ELF3. Proc Natl Acad Sci 109:19363–19367
Wang B, Gloer JB, Ji N, Zhao J (2013) Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem Rev 113:3632–3685
Wever R, van der Horst MA (2013) The role of vanadium haloperoxidases in the formation of volatile brominated compounds and their impact on the environment. Dalton Trans 42:11778–11786
Wever R, Krenn BE, Renirie R (2018) Marine vanadium-dependent haloperoxidases, their isolation, characterization, and application. Meth Enzymol 605:141–201
Weyand M, Hecht HJ, Kieß M, Liaud MF, Vilter H, Schomburg D (1999) X-ray structure determination of a vanadium-dependent haloperoxidase from Ascophyllum nodosum at 2.0 Å resolution. J Mol Biol 293:595–611
Wischang D, Hartung J (2011) Parameters for bromination of pyrroles in bromoperoxidase-catalyzed oxidations. Tetrahedron 67:4048–4054
Zhang Y-A, Yaw N, Snyder SA (2019) General synthetic approach for the Laurencia family of natural products empowered by a potentially biomimetic ring expansion. J Am Chem Soc 141:7776–7788
Acknowledgements
Kensuke Kaneko thanks the GCOE program of the Graduate School of Environmental Science at Hokkaido University. We thank Prof. Minoru Suzuki and Dr. Tsuyoshi Abe (Hokkaido University) for their valuable advice and suggestions. We are grateful to the staff of the Oshoro Marine Station, Field Science Center for Northern Biosphere, Hokkaido University, for their support and assistance during the field survey. We would like to thank Editage (www.editage.com) for English language editing.
Funding
This work was supported by a JSPS KAKENHI grant (No. 16H04975).
Author information
Authors and Affiliations
Contributions
Kensuke Kaneko conceived and designed experiments, conducted all experiments, and wrote the paper. Daiki Kobayashi, Shiro Masaki, and Kenji Washio cloned and heterologously expressed LsVBPO1. Masaaki Morikawa wrote and revised the manuscript accordingly. Tatsufumi Okino conceived and designed experiments, and wrote the manuscript. All authors have reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest regarding the publication of this paper.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaneko, K., Kobayashi, D., Masaki, S. et al. Gene cloning and characterization of a vanadium-dependent bromoperoxidase from the red alga Laurencia saitoi, a producer of brominated diterpenoids and triterpenoids. J Appl Phycol 35, 1443–1452 (2023). https://doi.org/10.1007/s10811-023-02953-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-02953-w