Abstract
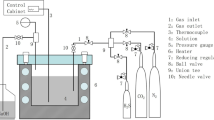
A H2S corrosion inhibition efficiency study by a carboxyamidoimidazoline compound was carried out using electrochemical impedance spectroscopy (EIS) and electrochemical noise (EN) techniques. The microalloyed steel assessed was specially designed for sour gas transport and is intended to be applied as line pipe steel in Mexico. The corrosive media was a deaerated 3% NaCl solution, H2S saturated after heated to 50 °C, with several inhibitor concentrations, ranging from 0 to 100 ppm, per test. The EIS and ENA data were analyzed and use as monitoring indicators for the development of a protective film by the inhibitor within 24 h testing. The results indicate that maximum inhibitor efficiency was achieved with only 5 ppm inhibitor concentration, but this efficiency value decreases with increasing of the inhibitor concentration. In addition the results also shows that the maximum efficiency was obtained at an elapsed time of 8 h, and beyond this time the efficiency again decrease. The EN transients prove that regardless the inhibitor concentration, the steel was highly susceptible to localized corrosion.














Similar content being viewed by others
References
Tesseder RS (1981) Oil industry experience with hydrogen embrittlement and stress corrosion cracking. In: Tuttle RN, Kane RD (eds) H2S corrosion in oil and gas production—a compilation of classic papers. NACE, Houston, p 147
Kermani MB, Morshed A (2003) Corrosion 59:659
Videm K, Kvarekval J (1995) Corrosion 51:260
Hong T, Sun YH, Jepson WP (2002) Corros Sci 44:101
Sastri VS (1998) Corrosion inhibitors: principles and applications. Willey, New York
Ashassi-Sorkhabi H, Nabavi-Amri SA (2002) Electrochim Acta 47:2239
Chetouani A, Aouniti A, Hammouti B, Benchat N, Benhadda T, Kertit S (2003) Corros Sci 45:1675
Singh I (1993) Corrosion 49:473
Ateya BG, EI-Anadouli BE, EI-Nizamy FM (1984) Corros Sci 24:497
Huang H-H, Lee J-T, Tsai W-T (1999) Mat Chem Phys 58:177
Ren C, Liu D, Bai Z, Li T (2005) Mat Chem Phys 93:305
Veloz MA, Gonzalez I (2002) Electrochim Acta 48:135
Garcia LACJ, Jois CBJM, Cardoso EM, Mattos DR (2001) Electrochim Acta 46:3879
Cabrera-Sierra R, Sosa E, Pech-Canul MA, Gonzalez I (2006) Electrochim Acta 51:1534
Galicia P, Gonzalez I (2005) Electrochim Acta 50:4451
Durnie W, De Marco R, Jefferson A, Kinsella BJ (1999) Electrochem. Soc 146:1751
Ramachandran S, Jovancicevic V (1999) Corrosion 55:259
Arzola S, Mendoza-Florez J, Duran-Romero R, Genesca J (2006) Corrosion 62:433
Jovancicevic V, Ramachandran S, Prince P (1999) Corrosion 55:449
Cruz J, Martinez R, Genesca J, Garcia-Ochoa EJ (2004) Electroanal Chem 566:111
Rodriguez-Valdez LM, Martinez-Villafañe A, Golztman-Mitnik D (2004) Theochem 681:83
Xue-Yuan Z, Feng-Ping W, Yu-Fang H, Yuan-Long D (2001) Corros Sci 43:1417
Olivares I, Alanis M, Mendoza R, Campillo B, Juarez-Islas JA (2008) Ironmak Steelmak 6:453
Gerus BRD (1981) H2S Corrosion in oil and gas production—a compilation of classic papers. NACE, Houston, p 888
Walter GW (1989) Corros Sci 26:681
Smith JS, Miller JDA (1975) Br Corros J 10:136
Gusmano G, Labella P, Montesperelli G, Privitera A, Tassinari S (2006) Corrosion 62:576
Rozenfel’d IL, Bogomolov DB, Gorodetskii AE, Kazanskii LP, Frolova LV, Shamova LI (1982) Protect Metals 18:121
French EC, Martin RL, Dougherty JA (1989) Mater Perform 28:46
Meyer FH, Riggs OL, McGlasson RL, Sudbury JD (1958) Corrosion 14:69
El-Awady AA, Abd-El-Nabey BA, Aziz SG (1992) J Electrochem Soc 139:2149
Acknowledgements
The authors acknowledge the financial support of Consejo Nacional de Ciencia y Tecnologia through “Programa de apoyo complementario para la consolidación institucional de grupos de investigacion” (Convocatoria 2006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Torres-Islas, A., Serna, S., Uruchurtu, J. et al. Corrosion inhibition efficiency study in a microalloyed steel for sour service at 50 °C. J Appl Electrochem 40, 1483–1491 (2010). https://doi.org/10.1007/s10800-010-0127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0127-5