Abstract
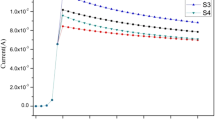
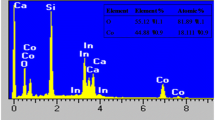
In order to control the amount of manganese oxide coated onto a graphite surface, immersion durations were varied. A maximum capacitance of 490 mF cm−2 was obtained in 0.5 M NaCl and using an immersion time of 20 min and a current of 1 mA. In addition, for the manganese oxide electrode, the lower the current, the higher the capacitance and the higher the immersion time, the higher the resistance. Furthermore, the chronopotentiometric (CP) charge–discharge curves were symmetrical and featured similar isosceles triangles, which demonstrate high electrochemical reversibility and good stability. Cyclic voltammograms of the manganese oxide electrode demonstrate that its operational stability is high.






Similar content being viewed by others
References
Conway BE (1999) Electrochemical supercapacitors—scientific fundamentals and technological applications. Kluwer Academic, New York
Kotz R, Carlen M (2000) Electrochim Acta 45:2483
Chen YS, Hu CC (2003) Electrochem Solid-State Lett 6:A210
Jeong YU, Manthiram A (2002) J Electrochem Soc 149:A1419
Hu CC, Wang CC (2003) J Electrochem Soc 150:A1079
Park HP, Park OO, Shin KH et al (2002) Electrochem Solid-State Lett 5:H7
Reddy RN, Reddy RG (2003) J Power Sources 124:330
Wu M, Snook GA, Chen GZ et al (2004) Electrochem Commun 6:499
Burke A (2000) J Power Sources 91:37
Chang JK, Lin CT, Tsai WT (2004) Electrochem Commun 6:666
Hong MS, Lee SH, Kim SW (2002) Electrochem Solid-State Lett 5:A227
Park JH, Ko JM, Park OO (2003) J Electrochem Soc 150:A864
Zhang JR, Chen B, Li WK et al (2002) Int J Mod Phys B 16:4479
Zheng JP, Jow TR (1995) J Electrochem Soc 142:L6
Zheng JP, Cygan PJ, Jow TR (1995) J Electrochem Soc 142:2699
Chang JK, Tsai WT (2003) J Electrochem Soc 150:A1333
Pang SC, Anderson MA, Thomas WC (2000) J Electrochem Soc 147:444
Lee HY, Kim SW, Lee HY (2001) Electrochem Solid-State Lett 4:A19
Pourbaix M (1966) Atlas of electrochemical equilibria in aqueous solutions. Pergamon Press, Brussels
Lin CC, Yen CC (2007) J Appl Electrochem 37(7):813
Wu BL, Lincot D, Vedel J, Yu LT (1997) J Electroanal Chem 420:159
Pang SC, Anderson MA (2000) J Mater Res 15(10):2096
Acknowledgement
Financial support by the National Science Council of the Republic of China (under grant no. NSC 93-2622-E-224-002-CC3) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, CC., Chen, HW. Electrochemical characteristics of manganese oxide electrodes prepared by an immersion technique. J Appl Electrochem 39, 1877–1881 (2009). https://doi.org/10.1007/s10800-009-9892-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-009-9892-4