Abstract
Purpose
Pharmacological treatments for age-related macular degeneration (ArMD) include anti-vascular endothelial growth factor therapies. Bevacizumab is used off-label, as it has no indication for ArMD. This study aims to identify and describe literature on real-world evidence of bevacizumab (originator or biosimilars) use in ArMD.
Methods
A scoping review was conducted in Medline, CINAHL and Embase databases. Studies published in English after September 2017, conducted in USA, including adults (≥ 18 years old) with ArMD who received treatment with bevacizumab for ArMD were included. The review was further limited to peer-reviewed observational studies that quantitatively analyze either clinical or patient-reported outcomes among patients treated with bevacizumab for ArMD.
Results
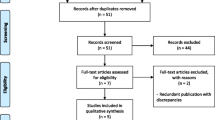
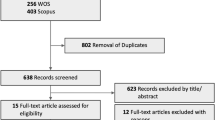
The search strategy retrieved 543 studies. After title and abstract screening, a total of 142 studies were selected for full-text review leading to a total of 12 studies qualifying for data charting. All were retrospective studies. Five (41.6%) of the studies had less than 500 eyes included in the analysis, and the rest had over a thousand eyes. All except one study reported clinical outcomes (visual acuity was the main outcome in 8 (66.6%) studies). There were 3 (25%) studies reporting adverse events of bevacizumab intravitreal injections. None of the studies specified using biosimilars for bevacizumab and none mentioned patient-reported outcomes.
Conclusion
The lack of studies aiming to study the patient-reported outcomes as well as the use of biosimilars of bevacizumab in ArMD makes this field a potential for future research. The different exposures and times to follow-up make it difficult to compare results among the selected studies.

Similar content being viewed by others
References
Mitchell P, Liew G, Gopinath B, Wong TY (2018) Age-related macular degeneration. The Lancet 392:1147–1159. https://doi.org/10.1016/S0140-6736(18)31550-2
Steinmetz JD, Bourne RRA, Briant PS et al (2021) Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Health 9:e144–e160. https://doi.org/10.1016/S2214-109X(20)30489-7
National Eye Institute. (2021) Age-Related Macular Degeneration (AMD) Data and Statistics. https://www.nei.nih.gov/learn-about-eye-health/outreach-campaigns-and-resources/eye-health-data-and-statistics#5
Kim S, Park SJ, Byun SJ et al (2019) Incremental economic burden associated with exudative age-related macular degeneration: a population-based study. BMC Health Serv Res 19:828. https://doi.org/10.1186/s12913-019-4666-0
Brown MM, Brown GC, Lieske HB et al (2016) Societal costs associated with neovascular age-related macular degeneration in the United States. Retina 36:285–298. https://doi.org/10.1097/IAE.0000000000000717
Flaxel CJ, Adelman RA, Bailey ST et al (2020) Age-related macular degeneration preferred practice pattern®. Ophthalmology 127:P1–P65. https://doi.org/10.1016/j.ophtha.2019.09.024
Heesterbeek TJ, Lorés-Motta L, Hoyng CB et al (2020) Risk factors for progression of age-related macular degeneration. Ophthalm Physiol Opt 40:140–170. https://doi.org/10.1111/opo.12675
Markham A (2019) Brolucizumab: first approval. Drugs 79:1997–2000. https://doi.org/10.1007/s40265-019-01231-9
Label F (2011) AVASTIN® (bevacizumab)
Bro T, Derebecka M, Jørstad ØK, Grzybowski A (2020) Off-label use of bevacizumab for wet age-related macular degeneration in Europe. Graefe’s Arch Clin Exper Ophthalmol 258:503–511. https://doi.org/10.1007/s00417-019-04569-8
Li E, Donati S, Lindsley KB et al (2020) Treatment regimens for administration of anti-vascular endothelial growth factor agents for neovascular age-related macular degeneration. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD012208.pub2
Holekamp NM (2019) Review of neovascular age-related macular degeneration treatment options. Am J Manag Care 25:S172–S181
U.S. Food & Drug Administration FDA-Approved Biosimilar Products. Biosimilar Product Information
van Asten F, Michels CTJ, Hoyng CB et al (2018) The cost-effectiveness of bevacizumab, ranibizumab and aflibercept for the treatment of age-related macular degeneration—A cost-effectiveness analysis from a societal perspective. PLoS ONE 13:e0197670. https://doi.org/10.1371/journal.pone.0197670
Singh RP (2018) American Society of Retina Specialists Preferences and trends Survey 2018. In: ASRS Global Trends in Retina. https://www.asrs.org/content/documents/2018-global-trends-in-retina-survey-highlights-website.pdf
CATT Research Group, Martin DF, Maguire MG et al (2011) Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med 364:1897–1908. https://doi.org/10.1056/NEJMoa1102673
Solomon SD, Lindsley K, Vedula SS et al (2019) Anti-vascular endothelial growth factor for neovascular age-related macular degeneration. Cochrane Database Syst Rev 3:CD005139. https://doi.org/10.1002/14651858.CD005139.pub4
Markus R, Liu J, Ramchandani M et al (2017) Developing the totality of evidence for biosimilars: regulatory considerations and building confidence for the healthcare community. BioDrugs 31:175–187. https://doi.org/10.1007/s40259-017-0218-5
Sheth J, Stewart M, Khatri M et al (2021) Changing trends in the use of anti-vascular endothelial growth factor (anti-VEGF) biosimilars: insights from the vitreoretinal society of india biosimilars of Anti-VEGF survey. Indian J Ophthalmol 69:352. https://doi.org/10.4103/ijo.IJO_2703_20
Slean GR, Hemarat K, Khurana RN, Stewart JM (2016) Conversion back to bevacizumab or ranibizumab for recurrent neovascular activity with aflibercept in age-related macular degeneration: a case series. Int J Retina Vitr 2:2. https://doi.org/10.1186/s40942-016-0028-9
Mirshahi A, Lashay A, Riazi-Esfahani H et al (2021) Intraocular injection of stivant® a biosimilar to bevacizumab: a case series. J Ophthalm Vis Res. https://doi.org/10.18502/jovr.v16i1.8248
Peters, M. D., Godfrey, C. M., McInerney, P., Soares, C. B., Khalil, H., & Parker D (2015) The Joanna Briggs Institute Reviewers’. Manual 2015: Methodology for JBI scoping reviews. Joanne Briggs Institute 1–24
Gomez-Lumbreras A, Giannouchos T, Panchal R, Ghule P, Lockhart DB CM (2021) Real-world evidence outcomes in the use of bevacizumab in age-related macular degeneration (ArMD): a scoping review protocol. Protocol Registry Evidence Rev Univ Utah 7:58. https://doi.org/10.26052/0d-b3vq-8d5m
Tricco AC, Lillie E, Zarin W et al (2018) PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 169:467–473. https://doi.org/10.7326/M18-0850
(2021) Covidence systematic review software. In: Veritas Health Innovation. Melbourne, Australia. www.covidence.org.
Adrean SD, Chaili S, Ramkumar H et al (2018) Consistent long-term therapy of neovascular age-related macular degeneration managed by 50 or more Anti–VEGF injections using a treat-extend-stop protocol. Ophthalmology 125:1047–1053. https://doi.org/10.1016/j.ophtha.2018.01.012
Eng VA, Rayess N, Nguyen HV, Leng T (2020) Complete RPE and outer retinal atrophy in patients receiving anti-VEGF treatment for neovascular age-related macular degeneration. PLoS ONE 15:e0232353. https://doi.org/10.1371/journal.pone.0232353
Parikh R, Ross JS, Sangaralingham LR et al (2017) Trends of anti-vascular endothelial growth factor use in ophthalmology among privately insured and medicare advantage patients. Ophthalmology 124:352–358. https://doi.org/10.1016/j.ophtha.2016.10.036
Hwang RY, Santos D, Oliver SCN (2020) Rates of exudative recurrence for eyes with inactivated wet afe-related macular degeneratio on 12-week interval dosing with Bevacizumabtherapy. Retina 40:679–685. https://doi.org/10.1097/IAE.0000000000002446
Kiss S, Dugel PU, Khanani AM et al (2018) Endophthalmitis rates among patients receiving intravitreal anti-VEGF injections: a USA claims analysis. Clin Ophthalmol 12:1625–1635. https://doi.org/10.2147/OPTH.S169143
Atchison EA, Wood KM, Mattox CG et al (2018) The real-world effect of intravitreous anti-vascular endothelial growth factor drugs on intraocular pressure: an analysis using the IRIS registry. Ophthalmology 125:676–682. https://doi.org/10.1016/j.ophtha.2017.11.027
Maloney MH, Payne SR, Herrin J et al (2021) Risk of systemic adverse events after intravitreal bevacizumab, ranibizumab, and aflibercept in routine clinical practice. Ophthalmology 128:417–424. https://doi.org/10.1016/j.ophtha.2020.07.062
Soares RR, Mellen P, Garrigan H et al (2020) Outcomes of eyes lost to follow-up with neovascular age-related macular degeneration receiving intravitreal anti-vascular endothelial growth factor. Ophthalmol Retina 4:134–140. https://doi.org/10.1016/j.oret.2019.07.010
Kröger J, Fasching P, Hanaire H (2020) Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Therapy 11:279–291. https://doi.org/10.1007/s13300-019-00741-9
Ghasemi Falavarjani K, Nguyen QD (2013) Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye 27:787–794. https://doi.org/10.1038/eye.2013.107
Porta M, Striglia E (2020) Intravitreal anti-VEGF agents and cardiovascular risk. Intern Emerg Med 15:199–210. https://doi.org/10.1007/s11739-019-02253-7
Okada M, Mitchell P, Finger RP et al (2021) Nonadherence or nonpersistence to intravitreal injection therapy for neovascular age-related macular degeneration. Ophthalmology 128:234–247. https://doi.org/10.1016/j.ophtha.2020.07.060
Martel A, Nahon-Esteve S, Martini K et al (2020) Feelings, preoperative anxiety, and need for information in patients undergoing intravitreal injections. Graefe’s Arch Clin Exper Ophthalmol 258:1395–1403. https://doi.org/10.1007/s00417-020-04699-4
Hasan H, Flockhart S, Qureshi W et al (2017) Intravitreal injections service: a patient experience evaluation. British J Nurs 26:678–682. https://doi.org/10.12968/bjon.2017.26.12.678
Gualino V, Fourmaux E, Grenet T et al (2020) Patient experience of anti-vegf intravitreal injection. J Français d’Ophtalmologie 43:1047–1053. https://doi.org/10.1016/j.jfo.2020.02.006
Ehlken C, Ziemssen F, Eter N et al (2020) Systematic review: non-adherence and non-persistence in intravitreal treatment. Graefe’s Arch Clin Exper Ophthalmol 258:2077–2090. https://doi.org/10.1007/s00417-020-04798-2
Pennington BM, Hernández-Alava M, Hykin P et al (2020) Mapping from visual acuity to EQ-5D, EQ-5D with vision bolt-on, and VFQ-UI in patients with macular edema in the LEAVO trial. Value Health 23:928–935. https://doi.org/10.1016/j.jval.2020.03.008
Michael Mezher (2015) French Regulator and Roche Trade Blows Over Off-Label Avastin Use. Regulatory Affairs Professionals Society 2
Davio K (2018) UK Health System Wins the Right to Treat AMD With Bevacizumab. In: The Center For Biosimilars AJMC. https://www.centerforbiosimilars.com/view/uk-health-system-wins-the-right-to-treat-amd-with-bevacizumab
Fogli S, Del Re M, Rofi E et al (2018) Clinical pharmacology of intravitreal anti-VEGF drugs. Eye 32:1010–1020. https://doi.org/10.1038/s41433-018-0021-7
Sharma A, Reddy P, Kuppermann BD et al (2018) Biosimilars in ophthalmology: is there a big change on the horizon? Clin Ophthalmol 12:2137–2143. https://doi.org/10.2147/OPTH.S180393
Zhang J, Liu Z, Hu S, Qi J (2020) Meta-analysis of the pharmacogenetics of ARMS2 A69S polymorphism and the response to advanced age-related macular degeneration. Ophthalm Res. https://doi.org/10.1159/000508738
Arlett P, Kjær J, Broich K, Cooke E (2022) Real-World Evidence in EU Medicines Regulation: Enabling Use and Establishing Value. Clin Pharmacol Ther 111:21–23. https://doi.org/10.1002/cpt.2479
FDA (2021) Considerations for the Use of Real-World Data and Real-World Evidence To Support Regulatory Decision-Making for Drug and Biological Products. U.S.A.
Hutton (2022) Outlook Therapeutics announces FDA has accepted BLA for bevacizumab-vikg as a treatment for wet AMD
(2019) Why Are Biosimilars Not Living up to Their Promise in the US? AMA Journal of Ethics 21:E668–678. https://doi.org/10.1001/amajethics.2019.668
Van de Wiele VL, Hammer M, Parikh R, et al (2022) Competition law and pricing among biologic drugs: the case of VEGF therapy for retinal diseases. Journal of Law and the Biosciences 9:. https://doi.org/10.1093/jlb/lsac001
Chen BK, Yang YT, Bennett CL (2018) Why Biologics and Biosimilars Remain So Expensive: Despite Two Wins for Biosimilars, the Supreme Court’s Recent Rulings do not Solve Fundamental Barriers to Competition. Drugs 78:1777–1781. https://doi.org/10.1007/s40265-018-1009-0
Rosenfeld PJ, Windsor MA, Feuer WJ et al (2018) Estimating Medicare and Patient Savings From the Use of Bevacizumab for the Treatment of Exudative Age-related Macular Degeneration. Am J Ophthalmol 191:135–139. https://doi.org/10.1016/j.ajo.2018.04.008
Association for Accesible Medicines (2020) Generic Drug & Biosimilars Access & Savings in the U.S. Report. 36
FDA (2022) FDA-Approved Biosimilar Products. https://www.fda.gov/drugs/biosimilars/biosimilar-product-information
Dickson SR, Kent T (2021) Association of Generic Competition With Price Decreases in Physician-Administered Drugs and Estimated Price Decreases for Biosimilar Competition. JAMA Network Open 4:e2133451. https://doi.org/10.1001/jamanetworkopen.2021.33451
Greene L, Singh RM, Carden MJ, et al (2019) Strategies for Overcoming Barriers to Adopting Biosimilars and Achieving Goals of the Biologics Price Competition and Innovation Act: A Survey of Managed Care and Specialty Pharmacy Professionals. Journal of Managed Care & Specialty Pharmacy 25:904–912. https://doi.org/10.18553/jmcp.2019.18412
Vogler S, Schneider P, Zuba M, et al (2021) Policies to Encourage the Use of Biosimilars in European Countries and Their Potential Impact on Pharmaceutical Expenditure. Frontiers in Pharmacology 12:. https://doi.org/10.3389/fphar.2021.625296
Munn Z, Peters MDJ, Stern C et al (2018) Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 18:1–7. https://doi.org/10.1186/s12874-018-0611-x
Lee A, Garg PG, Lyon AT, et al (2020) Long-term Outcomes of Treat and Extend Regimen of Anti-vascular Endothelial Growth Factor in Neovascular Age-related Macular Degeneration. J Ophthalmic Vis Res 15:331–340. https://doi.org/10.18502/jovr.v15i3.7452
Rao P, Lum F, Wood K, et al (2018) Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti-VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology 125:522–528. https://doi.org/10.1016/j.ophtha.2017.10.010
Ciulla TA, Hussain RM, Pollack JS, Williams DF (2020) Visual Acuity Outcomes and Anti-Vascular Endothelial Growth Factor Therapy Intensity in Neovascular Age-Related Macular Degeneration Patients: A Real-World Analysis of 49 485 Eyes. Ophthalmol Retina 4:19–30. https://doi.org/10.1016/j.oret.2019.05.017
Kiss S, Campbell J, Almony A, et al (2020) Management and Outcomes for Neovascular Age-Related Macular Degeneration: Analysis of United States Electronic Health Records. Ophthalmology 127:1179–1188. https://doi.org/10.1016/j.ophtha.2020.02.027
Funding
The authors declare that no funds, grants or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AGL participated in developing the study protocol and research design, was one of the reviewers of the manuscripts, provided the search strategy, charted and analyzed data, contributed to interpretation the data, and drafted the initial manuscript. PG reviewed the provided list of manuscripts from the search strategy, charted and analyzed data, contributed to interpretation the data, and reviewed the manuscript. RP and TG participated in developing the study protocol and research design, contributed to interpretation the data, and edited and reviewed the manuscript. DB and CL supervised the study, drafted the study protocol and research design, guided the analysis of the data, provided interpretation, and reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This scoping review has been conducted using previously collected data. No need of informed consent or IRB approval was needed.
Consent to participate
Participants consent was not request as the study has been conducted using already collected and anonymous data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gomez-Lumbreras, A., Ghule, P., Panchal, R. et al. Real-world evidence in the use of Bevacizumab in age-related macular degeneration (ArMD): a scoping review. Int Ophthalmol 43, 4527–4539 (2023). https://doi.org/10.1007/s10792-023-02853-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02853-5