Abstract
Purpose
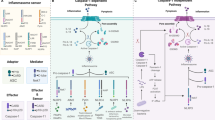
Pyroptosis is a newly discovered form of programmed pro-inflammatory cell death. The main signaling pathways include the classical scorch death pathway that depends on NLRP3 inflammatory vesicles and other activation caspase-1 and the non-classical scorch death pathway that depends on caspase-4 /5/11. The substrate of all inflammatory caspases is GSDMD; a large number of studies have confirmed that pyroptosis is associated with certain infectious diseases, atherosclerotic diseases, metabolic diseases, and aseptic inflammatory diseases of important organs. In recent years, pyroptosis has been studied partially in the ocular field. So, this article reviews the recent literature intending to help readers understand the main mechanisms of cellular scorch death and the progress of GSDMD-mediated cellular scorch death in retinal vascular inflammatory diseases.
Method
A detailed review of the literature related to pyroptosis and inflammatory diseases of the retinal vasculature is presented. The following 6 electronic databases were searched: CNKI, Wanfang, VIP, PubMed, The Cochrane Library, and Embase Databases, and the search period was from the database to May 2022. The main search keywords include “Pyroptosis,” “ GSDMD,” “Retinal Vascular Inflammatory Disease,” “Diabetic retinopathy,” “Retinal vasculitis.” The discovery of pyroptosis, the main molecular mechanisms, key proteins, and their pathogenesis and therapeutic prospects in retinal vasculitis diseases are extensively studied and summarized.
Result
The mechanisms of gasdermin D-mediated pyroptosis are elaborated and analyzed, with particular emphasis on their key role and potential in the pathogenesis and treatment of inflammatory retinal vascular lesions.
Conclusion
Gasdermin D-mediated pyroptosis is a well-studied form of programmed pro-inflammatory cell death, which has a bidirectional regulatory effect on a variety of immune and inflammatory diseases. The literature reveals that pyroptosis is closely related to the pathogenesis of retinal vascular inflammatory diseases, and it may be an important therapeutic target for diabetic retinopathy and other retinal vasculitis eye diseases in the future.

Similar content being viewed by others
References
Cookson B, Brennan M (2001) Pro-inflammatory programmed cell death. Trends Microbiol 9(3):113–114
Vande Walle L, Lamkanfi, (2016) Pyroptosis. Curr Bio 26(13):R568–R572
Ross C, Chan A, von Pein J et al (2022) Inflammatory Caspases: Toward a Unified Model for Caspase Activation by Inflammasomes. Annu Rev Immunol 40:249–269
Shi J, Zhao Y, Wang K et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526(7575):660–665
Kesavardhana S, Malireddi R, Kanneganti TJAroi, (2020) Caspases in Cell Death, Inflammation, and Pyroptosis. Annu Rev Immunol 38:567–595
Rumpret Matevž, von Richthofen Helen J, Peperzak Victor et al (2022) Inhibitory pattern recognition receptors. J Experimet Med. https://doi.org/10.1084/jem.20211463
Horvath G L, Schrum J E, De Nardo C M et al (2011) Intracellular sensing of microbes and danger signals by the inflammasomes: Inflammasome activation in inflammation. Immunological Reviews 243(1):119–135. https://doi.org/10.1111/j.1600-065X.2011.01050.x
Lu F, Lan Z, Xin Z et al (2020) Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases. J Cell Physiol 235(4):3207–3221
Man S M, Karki R, Kanneganti T-Devi (2017) Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol Rev 277(1):61–75. https://doi.org/10.1111/imr.12534
Chen X, He W, Hu L et al (2016) Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res 26(9):1007–1020
Kayagaki N, Wong M, Stowe I et al (2013) Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341(6151):1246–1249
Qiu S, Liu J, Xing F (2017) “Hints” in the killer protein gasdermin D: unveiling the secrets of gasdermins driving cell death. Cell death differs 24(4):588–596
Feng Shouya, Fox Daniel, Man Si Ming (2018) Mechanisms of Gasdermin Family Members in Inflammasome Signaling and Cell Death. J Molecul Biol 430(18):3068–3080. https://doi.org/10.1016/j.jmb.2018.07.002
Xia S (2020) Biological mechanisms and therapeutic relevance of the gasdermin family. Mol Aspects Med 76:100890
Broz P (2015) Immunology: Caspase target drives pyroptosis. Nature 526(7575):642–643
Saeki N, Usui T, Aoyagi K et al (2009) Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes chromosomes cancer 48(3):261–271
Burgener S, Leborgne N, Snipas S et al (2019) Cathepsin G Inhibition by Serpinb1 and Serpinb6 Prevents Programmed Necrosis in Neutrophils and Monocytes and Reduces GSDMD-Driven Inflammation. Cell Rep 27(12):3646-3656.e3645
Kambara H, Liu F, Zhang X et al (2018) Gasdermin D Exerts Anti-inflammatory Effects by Promoting Neutrophil Death. Cell Rep 22(11):2924–2936
Xi G, Gao J, Wan B et al (2019) GSDMD is required for effector CD8 T cell responses to lung cancer cells. Int Immunopharmacol 74:105713
Liu X, Zhang Z, Ruan J et al (2016) Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535(7610):153–158
Liu Z, Wang C, Rathkey J et al (2018) Structures of the Gasdermin D C-Terminal Domains Reveal Mechanisms of Autoinhibition. Structure 26(5):778-784.e773
Liu Z, Wang C, Yang J et al (2019) Crystal Structures of the Full-Length Murine and Human Gasdermin D Reveal Mechanisms of Autoinhibition, Lipid Binding, and Oligomerization. Immunity 51(1):43-49.e44
Ding J, Wang K, Liu W et al (2016) Erratum: Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 540(7631):150
Teng Jin-Feng, Mei Qi-Bing, Zhou Xiao-Gang et al (2020) Polyphyllin VI Induces Caspase-1-Mediated Pyroptosis via the Induction of ROS/NF-κB/NLRP3/GSDMD Signal Axis in Non-Small Cell Lung Cancer. Cancers 12(1):193. https://doi.org/10.3390/cancers12010193
Rathkey J, Zhao J, Liu Z, et al (2018) Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol, 3(26).
Bansal N, Sciabola S, Bhisetti G (2019) Understanding allosteric interactions in hMLKL protein that modulate necroptosis and its inhibition. Sci Rep 9(1):16853
Feenstra D, Yego E, Mohr S (2013) Modes of Retinal Cell Death in Diabetic Retinopathy. J Clin Exp Ophthalmol 4(5):298
Petersen L, Bek T (2019) The Oxygen Saturation in Vascular Abnormalities Depends on the Extent of Arteriovenous Shunting in Diabetic Retinopathy. Invest Ophthalmol Vis Sci 60(12):3762–3767
Simó R, Hernández C, (2014) Neurodegeneration in the diabetic eye: new insights and therapeutic perspectives .Trends Endocrinol Metab , 25(1):23–33.
Ferrington DA, Fisher CR, Kowluru RA (2020) Mitochondrial Defects Drive Degenerative Retinal Diseases. Trends Mol Med 26(1):105–118
Chai G, Liu S, Yang H et al (2020) NLRP3 Blockade Suppresses Pro-Inflammatory and Pro-Angiogenic Cytokine Secretion in Diabetic Retinopathy. Diabetes, metabolic syndrome, and obesity: targets and therapy 13:3047–3058
Loukovaara S, Piippo N, Kinnunen K et al (2017) NLRP3 inflammasome activation is associated with proliferative diabetic retinopathy. Acta Ophthalmol 95(8):803–808
Yin Y, Chen F, Wang W et al (2017) Resolvin D1 inhibits inflammatory response in STZ-induced diabetic retinopathy rats: Possible involvement of NLRP3 inflammasome and NF-κB signaling pathway. Mol Vis 23:242–250
Zhang Y, Lv X, Hu Z et al (2017) Protection of Mcc950 against high-glucose-induced human retinal endothelial cell dysfunction. Cell Death Dis 8(7):e2941
Solini A, Novak I (2019) Role of the P2X7 receptor in the pathogenesis of type 2 diabetes and its microvascular complications. Curr Opin Pharmacol 47:75–81
Lu L, Lu Q, Chen W et al (2018) Vitamin D Protects against Diabetic Retinopathy by Inhibiting High-Glucose-Induced Activation of the ROS/TXNIP/NLRP3 Inflammasome Pathway. J Diabetes Res 2018:8193523
Liu Q, Zhang F, Zhang X et al (2018) Fenofibrate ameliorates diabetic retinopathy by modulating Nrf2 signaling and NLRP3 inflammasome activation. Mol Cell Bio 445:105–115
Sun H, Jin X, Xu J et al (2020) Baicalin Alleviates Age-Related Macular Degeneration via miR-223/NLRP3-Regulated Pyroptosis. Pharmacol 105:28–38
Yu X, Ma X, Lin W, et al(2020)Long noncoding RNA MIAT regulates primary human retinal pericyte pyroptosis by modulating miR-342–3p targeting of CASP1 in diabetic retinopathy. Exp Eye Res:108300.
Datoo GA, O’Keefe N R (2021) Retinal vasculitis: A framework and proposal for a classification system. Survey of Ophthalmology 66(1):54–67. https://doi.org/10.1016/j.survophthal.2020.05.004
Rosenbaum J, Sibley C, Lin P (2016) Retinal vasculitis. Cur Opinion. Rheumatol 28(3):228–235
Sui A, Chen X, Shen J et al (2020) Inhibiting the NLRP3 inflammasome with MCC950 ameliorates retinal neovascularization and leakage by reversing the IL-1β/IL-18 activation pattern in an oxygen-induced ischemic retinopathy mouse model. Cell Death Dis 11(10):901
Acknowledgements
Thanks go to the corresponding author who provided valuable suggestions for revision of this review.
Funding
LX is funded by the National Nature Science Foundation of China (No. 81473735). The corresponding author is the sponsor who provides for funds.
Author information
Authors and Affiliations
Contributions
LX contributed to the conception and design of the study, data collection, analysis, interpretation of data, and drafting of the manuscript. Supervision was performed by X-X. All authors contributed to the critical revision of the manuscript for important intellectual content. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiaodong, L., Xuejun, X. GSDMD-mediated pyroptosis in retinal vascular inflammatory diseases: a review. Int Ophthalmol 43, 1405–1411 (2023). https://doi.org/10.1007/s10792-022-02506-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02506-z