Abstract
Dry eye syndrome (DES) is multifactorial and likely to be a cause of concern more so than ever given the rapid pace of modernization, which is directly associated with many of the extrinsic causative factors. Additionally, recent studies have also postulated novel etiologies that may provide the basis for alternative treatment methods clinically. Such insights are especially important given that current approaches to tackle DES remains suboptimal. This review will primarily cover a comprehensive list of causes that lead to DES, summarize all the upcoming and ongoing clinical trials that focuses on treating this disease as well as discuss future potential treatments that can improve inclusivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
DES is a relatively common clinical ophthalmic condition, characterized by a disorder of the preocular tear film and affecting approximately 1 out of 7 individuals aged 48 and above [1]. Besides DES, this condition can also be known as keratoconjunctivitis sicca (KCS), dry eye disease (DED), ocular surface disease (OSD) or dysfunctional tear syndrome (DTS) [2]. It is a dysfunction of the nasolacrimal unit (nasolacrimal glands, corneal surface and eyelids) which leads to defective or insufficient tear film formation [3].
The maintenance of a physiologically complete tear film is imperative for normal vision as it is, along with the cornea, responsible for focusing light onto the retina [4]. Additionally, it also functions to lubricate the eye, remove debris from the ocular surface as well as maintain nutrition and oxygenation of the ocular structures [5]. Patients who developed DES may experience ocular burning, blurred vision or even pain and often have a reduced quality of life as common daily tasks that require visual attention (e.g. reading, computer work, etc.) become significantly challenging. However, while treatments are available to minimize the negative impacts, they are often suboptimal and unable to specifically target the root cause(s) of this disease.
It is now known that DES can be caused by a non-exhaustive list of factors which include autoimmunity, hormonal imbalance, deleterious environmental settings and many more. Unbeknown to many, symptoms associated with dry eyes may even at times be indicative of undiagnosed systemic diseases which, if treated timely, may avoid life-threatening outcomes [5]. Over the years, given a more profound understanding of the various mechanisms involved in the development of this condition, a wide range of novel treatments are underway to provide more effective results and overcome limitations posed by conventional therapeutics utilized in the clinic currently. This review aims to summarize the causes of DES and its respective mechanism, explore ongoing clinical trials for DES treatment and lastly, discuss promising technologies that can potentially shape future treatment strategies.
Secretory components and tear film composition
The tear film is regulated by an integrated lachrimal functional unit (LFU) which consists of the lachrimal glands, cornea, conjunctiva, eyelids, meibomian glands, goblet cells as well as the sensory and motor nerves that connect them [6]. As measured by ultrahigh resolution optical coherence tomography (OCT) and validated with interferometry techniques [7, 8], it was found that the tear film, when spread across the exposed conjunctiva and cornea, is approximately 2 to 5.5 μm thick [9]. Correspondingly, this extremely thin layer of film is constituted by an even thinner top layer of lipid (about 42 nm) [10] and a mucin-aqueous (mucoaqueous) layer with decreasing concentration of mucins from the cornea epithelium towards the lipid layer [11, 12].
The lipid layer is derived from meibum produced by the meibomian gland and secreted through the lid margins. Meanwhile, blinking helps to spread the lipid layer across the tear film through surface tension forces. This configuration functions to stabilize the film by preventing the aqueous component from evaporating too rapidly [13,14,15]. The aqueous fluid in the tear film, which contains water, electrolytes, small molecule metabolites, plethora of proteins (more than 1500 detected [16]) and peptides (more than 200 [17]) is mostly produced by the lacrimal glands. The aqueous portion in the mucoaqueous layer provides oxygen and nutrients to the underlying avascular corneal tissue and assist in flushing away epithelial debris, toxins and foreign bodies [18].
Secreted mucins are present in the aqueous component as well and are produced by the goblet cells present in the conjunctiva while transmembrane mucins (glycocalyx) that can extend up to 500 nm from the plasma membrane are formed on the apical surfaces of the corneal and conjunctival epithelia [19]. Mucins are large high molecular weight glycoproteins that contain one of more protein domains which are rich in serines and threonines extensively glycosylated via O-glycan attachments [20, 21]. They are essential for providing lubrication, hydration as well as protection against infection and injury [22, 23]. On the ocular surface, it was shown recently that they further maintain a disadhesive property to the apical epithelial cells such that, during blinking or sleeping, cell surfaces facing each other like the cornea and conjunctiva do not adhere to each other [24]. Together, these constituents maintain the tear film and any slight dysregulation such as decreased aqueous volume or abnormal production of mucins or lipids will lead to DES [25, 26].
Etiology of DES
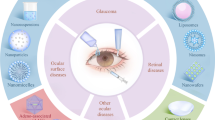
There are a multitude of factors that have been discovered to result in such dysregulations which, in general, can be classified as intrinsic and extrinsic. Intrinsic factors are defined as conditions present within the body and include autoimmunity [3, 27, 28], hormonal imbalance [29, 30], systemic diseases [31,32,33,34], hereditary diseases [35, 36], nerve damage [37, 38] and gut dysbiosis [39, 40]. On the other hand, extrinsic elements are derived from stimulus that occur outside the body and consist of environmental influences [41, 42], behaviour and/or habits [43,44,45,46], eye accessories [47] and eye surgeries [48, 49] (Fig. 1).
Intrinsic factors
Autoimmunity
Dry eyes caused by autoimmunity could be attributed to Sjörgen’s syndrome (SS), a chronic autoimmune disorder that primarily affects the salivary and lacrimal glands. Specifically, these exocrine glands are heavily infiltrated with lymphocytes (T cells and B cells) and macrophages which produces pro-inflammatory signalling molecules such as IL-1, TNF-α and IFN-γ [28, 50,51,52]. CD4+ T cells are the primary immune effectors [53] and interact closely with antigen presenting-macrophages to provoke ocular disease development through inflammation-induced (IL-1 and IFN-γ) local tissue damage [51]. Additionally, they are also associated with peripheral neuropathy in the lacrimal glands, suggesting possible denervation and loss of function [54]. Besides CD4+ T cells, it was recently observed that highly cytotoxic activated CD8+ T cells are correlated with lacrimal gland epithelial cell death [50] and may account for the reduction in tear production. Given the varied possible causes of DES, the diagnosis of SS-induced dry eyes is relatively tedious and requires defined biomarkers for validation. Accordingly, it is known that the tear film of patients who developed SS contained elevated amounts of pro-inflammatory cytokines such as IL-1, IL-6, IL-8 and TNF-α. Their presence also corresponded to lower tear secretion levels [55,56,57]. Other biomarkers include MMP-9 [58, 59], HLA-DR [60, 61] and potentially MUC5AC [62].
Graves’ opthalmopathy, also known as thyroid eye disease, is another autoimmune condition that can lead to DES [63]. Patients afflicted with this disease produce excessive thyroid hormones which induce an inflammatory response in the orbital tissues [64]. Mechanistically, DES is caused by a combination of mechanical impairment of the lids [65] and autoantibodies targeting the thyroid-stimulating hormone receptors on the lacrimal gland [66]. Incomplete blinking due to lid impairment results in inadequate tear distribution over the ocular surface and excessive tear evaporation [65] while autoantibodies binding causes aberrant signal transduction in the lacrimal gland and subsequent tear hyposecretion [66].
While not commonly known, multiple sclerosis, where the central nervous system (CNS) becomes demyelinated, is also an autoimmune disease that is correlated to DES. Specifically, poor corneal sensory impulse conduction due to demyelination can lead to insufficient tear production [67].
Hormonal imbalance
Hormones are known to influence both the lacrimal and meibomian glands [68]. Sex hormones, particularly androgens, appeared to account for many of the sex-related disease susceptibility of the lacrimal gland in a variety of species [69]. For instance, testosterone was able to upregulate and downregulate a substantial amount of lacrimal gland genes found to be highly and lowly expressed respectively in male vs. female mice [70, 71]. On the other hand, estrogen and progesterone only impacted a small percentage [71] of these differentially expressed genes between male and female mice [70]. Mechanistically, androgens have been demonstrated to regulate the lacrimal glands’ fluid and protein secretion [72,73,74] through saturable, high-affinity and steroid-specific receptors binding in acinar and ductar epithelial cells [69, 75]. Accordingly, the lack of androgens was linked to lacrimal gland dysfunction and corresponding aqueous tear deficiency [30, 76], which helps to explain the higher DES prevalence among females [77, 78] since they are prone to reduced serum androgen levels during various stages of their life (lactation and menopause) [79, 80]. The meibomian glands, which are sebaceous in nature and contain acinar epithelial cells with androgen receptors, are also regulated by androgens [81, 82]. This form of regulation is dependent on 5α-reductase, an enzyme crucial for the production of the potent androgen, 5α-dihydrotestosterone (DHT). In the presence of DHT, these acinar cells display enhanced synthesis and secretion of lipids. Conversely, a reduction in DHT resulted in attenuated gland activity, size and lipid release [82, 83], which, in the context of DES, leads to the formation of an unstable tear film attributable to the increased rate of evaporation.
Systemic diseases
Diabetes mellitus (DM) is regarded as one of the leading systemic risk factors for DES due to the high prevalence (~ 18% to 54%) observed in Type 2 diabetic patients [84,85,86]. However, regardless of Type 1 or 2 diabetes, both conditions heighten the risk of developing LFU dysfunction such as corneal and conjunctival epithelium damage due to increased levels of HbA1c in blood serum [87]. HbA1c are glycated haemoglobins and provide an estimate of the blood sugar levels of an individual over the last three months [88]. As the conjunctiva epithelium contains goblet cells, the damage sustained will also be associated with diminished mucin production. Additionally, hyperglycemia has been shown to activate aldose reductase, an enzyme that catalyzes the conversion of glucose to the cytotoxic sorbitol [89]. Correspondingly, elevated amounts of sorbitol within cells will lead to cellular apoptosis and ultimately lacrimal gland structure dysfunction followed by the reduction in tear secretion [31].
Xerophthalmia is a systemic disease that consists of a variety of eye disorders, including DES [90]. It is attributed to vitamin A deficiency and is the only vitamin deficiency disease in the world that causes major concern to the public health personnel [91, 92]. Vitamin A is crucial for maintaining the differentiation and proliferation of the conjunctiva and corneal epithelium [93] by inhibiting the upregulation of apoptotic signals [94]. The lack of vitamin A will therefore lead to loss of goblet cells and mucin production.
Hereditary diseases
Familial dysautonomia (FD), also known as Riley-Day syndrome, is a rare, hereditary autosomal recessive disorder that impairs the development of specific sensory and autonomic neurons during embryogenesis [35]. As a result of this maldevelopment, patients with FD are highly vulnerable to optic neuropathy during their childhood, which becomes worse as they age [36]. Without proper control of their LFU, they lack the ability to produce tears at a basal, reflex and emotional level [95].
Nerve damage
All the secretory functions in the LFU are regulated by autonomic nerves. The lacrimal gland is largely innervated by the Vasoactive Intestinal Peptide immunoreactive (VIP-IR) parasympathetic nerve fibers (secretory control) [96, 97] and, to a lesser extent, sympathetic nerve fibers (vasculature control) immunoreactive to Neuropeptide Y (NPY-IR), Tyrosine Hydroxylase (TH-IR) and Dopamine β-Hydroxylase (DBH-IR) [98]. Upon stimulation, water and electrolytes, supplied by the blood, are transported into the duct system by the coordinated activation of ion channels and pumps [99,100,101]. Meanwhile, proteins produced and stored in the secretory granules of the lacrimal gland acinar cells will be released through stimulus-induced exocytosis [102] and carried along with the ionic fluid.
The meibomian gland and goblet cells in the conjunctiva is regulated by both parasympathetic VIP-IR and sympathetic DBH-IR and NPY-IR nerve fibers as well [97, 103, 104]. VIP-IR nerve fibers are located in close proximity to the acini and central duct of the meibomian gland where they influence the secretion of lipids, contributed by the meibocyte acinar cells, into the lumen of the duct system [97, 105]. On the other hand, VIP-IR nerve fibers are located at the epithelial-stroma junction in the conjunctiva, near the basal membrane of the goblet cells [104]. Upon receiving an appropriate stimulus, the secretory granules within the goblet cells fuse with each other and with the apical membrane to release the mucins, along with some amount of water and electrolytes, onto the ocular surface.
Correspondingly, the activity of these autonomic nerves are dependent on reflexes initiated by the activation of sensory neurons, which are present in high density, on the ocular surface [38]. At that location, they are very susceptible to direct injury caused by environmental factors and mechanical trauma [38]. Indirect forms of injury can also occur. For example, patients with aqueous tear deficiency from other causes may blink too frequently, which can generate enough stress to damage terminal nerve branches. Besides that, inflammation also plays a key role in altering the physiological state of the peripheral sensory neurons. Specifically, pro-inflammatory signalling molecules are able to either reduce the sensory neurons’ threshold for activation (sensitization) or increase their ongoing nerve activity (excitation) [106]. Such changes are linked to the kinetics of the transduction ion channels and voltage-gated ion channels in the axonal membrane [107], affecting the generation and propagation of action potentials [108, 109]. Without consistent control over the activation of the autonomic nerve fibers, tear production will therefore be defective.
Gut dysbiosis
The human body is host to trillions of microbiota. Among the various regions, such as the oral cavity, respiratory tract, skin and gastrointestinal tract, that harbor these microorganisms [110], the colon is the organ which consists of the densest number of microbes [111]. This additional diversity of microbiome serves as a functional expansion of host genomes [112] and produces signaling molecules that facilitate host metabolism and regulation of host physiology [113]. Studies have revealed correlations between gut dysbiosis, defined as an imbalance of the gut microbiota diversity (disturbed or inversed Firmicutes/Bacteroidetes ratio), and DES. Specifically, this connection was hypothesized to occur through the gut dysbiosis-ocular surface-lacrimal gland axis which consists of five proposed immune-related mechanisms describing how ratio changes of gut commensal can lead to DES [114, 115]. For example, one of the mechanisms proposed involve the migration of gut dysbiosis-activated CD103+ or CXCR1+ dendritic cells or monocytes/macrophage to the ocular surface where they prime T cells to secrete pro-inflammatory cytokines in the ocular surface and lacrimal glands [114]. Consequently, the immune response mounted will lead to a decrease in goblet cells and acinar cells in the conjunctiva and lacrimal glands, respectively, resulting in reduced mucin and tear secretion.
Extrinsic factors
Environmental influences
The LFU is well-equipped to withstand tolerable amounts of impurities in the environment and prevent ocular surface damage through tear secretion. However, the protection provided by the tear film can be eroded if the pollution becomes too overwhelming, especially if it affects the function of the various secretory components in the LFU. Particulate matter smaller than 2.5 and 10 μm (PM2.5 and PM10), which consists of inorganic dust, dirt, soot particles and organic allergens like pollen grains, mold and microbial colonies, are common pollutants associated with DES [116,117,118,119,120,121]. Excessive and prolonged exposure of these pollutants to the ocular surface were shown to trigger chronic inflammatory responses and induce oxidative stress, both of which have cytotoxic effects on the secretory cells [41]. Similarly, gaseous pollutants such as NO2, SO2, O3 and volatile organic compounds (VOCs) such as formaldehyde, toluene and acetone were all found to be positively correlated with DES through inflammatory and cytotoxic causes [122,123,124,125].
Even in the absence of impurities and reactive gases, constant exposure to extreme environmental conditions such as strong winds, low humidity, high temperature and high altitude can directly affect ocular health as well [125,126,127]. These scenarios reduce the tear film stability and cause faster tear evaporation [41], resulting in DES.
Behaviour and/or habits
Tobacco consumption is one of the main causes of morbidity and mortality globally and has been associated with a number of systemic disorders and conditions, including DES [43,44,45]. Besides conventional cigarettes, battery-powered electronic cigarettes (ECs), which deliver nicotine through a heated vapor [128], are also recently shown to increase the risk of developing dry eyes [129]. Accordingly, both types of cigarettes affect ocular functionality through the smoke and/or combustion by-products produced, leading to inflammation and subsequent decreased quantity and quality of tear secretion as well as ocular surface damage [129, 130].
Additionally, long-term usage of computer, tablet and cell phone can also result in DES [131]. It was observed that users blink less when using such display devices with a screen, which prevented the formation of a stable tear film and therefore leading to a faster rate of tear evaporation [46].
Eye accessories
Contact lenses provide an aesthetic means for ocular refractive error correction over glasses and an estimated 140 million people in the world use them [132]. This estimation has remained relatively consistent over the past decade despite numerous improvements in contact lens technology [133]. Correspondingly, a major reason for this observation arises from eye discomfort, mostly the sensation of dry eyes, after prolonged usage [134]. Specifically, the close proximity of contact lens to the ocular surface poses a host of issues to the LFU and the tear film.
When fitted correctly, the contact lens cover the cornea completely and extends by ~ 2 mm onto the conjunctiva. In this configuration, every blink will cause it to move along the conjunctiva, which induces mechanical friction and goblet cells damage within the epithelium [135] over time [136, 137]. A reduction in goblet cell density will therefore lead to decreased mucin production and secretion, which affects tear film spreading. Besides that, contact lenses have also been associated with the loss of the meibomian gland and its orifice obstruction, resulting in impeded lipid synthesis and their transport to the tear film. Together, these dysregulations reduce the stability of the pre-lens tear film (PrLTF), the thin layer of fluid constrained between the cornea and the contact lens which is half the thickness of the normal pre-corneal tear film [138], and cause it to be susceptible to rapid evaporation, rupture and dry spot formation [139]. The lack of a consistent PrLTF is therefore a manifestation of DES.
Eye surgeries
Surgical procedures for ocular refractive errors such as laser-assisted in situ keratomileusis (LASIK), photorefractive keratectomy (PRK) and small incision lenticule extraction (SMILE) are recognized risk factors for developing dry eye [48, 49, 140]. This is attributable to a limitation posed by these surgical procedures where the sensory nerves present on the ocular surface will inevitably get damaged [48, 49, 140]. Without reliable sensory detection, the corneal sensation become impaired, which decreases basal and reflex tearing as well as rate of blinking [141,142,143]. Moreover, sensory denervation will also disrupt tear production by the lacrimal gland, leading to reduced tear secretion [144]. In addition to nerve damage, these refractive surgeries are also known to inflict damage to the conjunctival goblet cells [145,146,147]. Consequently, a reduction in goblet cell density signifies reduced mucin production and therefore, reduced tear film stability. Inflammatory responses induced as a result of postoperative wound-healing process is the last contributing factor to DES.
Altogether, these factors constitute the major known causes of DES. For clarity, they are compiled and summarized in Table 1.
Upcoming clinical trials for DES treatment
Method of search
A primary search was conducted using ClinicalTrials (http://clinicaltrials.gov) and the key words used were dry eye, keratoconjunctivitis sicca, dryness, ocular, ophthalmic and optic. The search filters ‘Not yet recruiting’, ‘Recruiting’, ‘Enrolling by invitation’ and ‘Active, not recruiting’ were then applied to sieve out all the upcoming clinical trials related to these keywords. From there, the studies were reviewed and included only if they are associated with DES treatment.
Search results
All the pending and current clinical trials that focused on DES treatment were compiled and tabulated in Table 2. Applying the ‘Not yet recruiting’ filter yielded a total of 47 studies, of which 14 were relevant for this review. For the ‘Recruiting’ filter, there were a total of 146 studies and 34 of them were relevant. Meanwhile, the ‘Enrolling by invitation’ filter provided a total of 10 studies and 5 of them were found to be relevant. Lastly, the ‘Active, not recruiting’ filter returned a total of 23 studies and 7 of them were screened to be relevant.
For each of these filter categories, the studies-of-interest were further grouped according to various treatment types such as biologic, drug, device, drug delivery system, dietary supplement, physical activity, combinatorial as well as unknown. Here, biologics are distinct from drugs and defined as large complex biological molecules or a combination of molecules that can be derived from carbohydrates, lipids, proteins, nucleic acids, whole cells and even tissues. On the other hand, drugs are designated as molecules that are synthesized chemically and have well-characterized molecular structures. Based on the classification, around 50% of the recent clinical trials will be utilizing biologics and drugs for DES treatment. These therapeutics vary greatly and will mostly be concocted into a solution for delivery as eye drops. Among them, cyclosporine will be one of the most commonly tested anti-inflammatory drug. Even though cyclosporine was already approved for use in clinics to treat DES [207, 208], many of these studies are attempting to further improve its potency by testing different concentrations (NCT04835623), duration (NCT04144413) and delivery method such as sustained release (NCT04541888) and nanoencapsulation (NCT04172961). On the other hand, hyaluronic acid and its salt derivative, sodium hyaluronate, will be the most popular biologics utilized in these studies to constitute artificial tears with lubricating [156, 209] and antibacterial properties [210, 211]. As they were also FDA approved, improvements included the addition of other dietary supplements (NCT04485533) and lubricants (NCT03697876).
Besides biologics and drugs, medical devices are also quite commonly employed, especially for DES caused by Meibomian Gland Dysfunction (MGD). These commercial devices such as TearCare System (NCT04309799, NCT04795752) and MiBo Thermoflo (NCT03767530) usually have components that are attached to the users’ eyelids for providing heat and/or pressure which enhances meibum lipid flow [162, 212]. For other causes of DES, one study will be investigating the efficacy of quantum molecular resonance (QMR) on patients with DES (NCT04320563). QMR is a recent innovative technology that involves the application of low-power high-frequency oscillating electrical currents (4 to 64 MHz), a range which resonates with biological tissues, in order to elicit cellular responses [213]. This procedure will be performed using Rexon-Eye, a noninvasive, QMR-based patented instrument. Patients will wear the device like an eye mask and electrodes will stimulate their periorbital region during the therapy for enhanced tear secretion.
The rest of the treatment types form the minority within the list of clinical trials. These included drug delivery systems that will provide sustained release through dexamethasone-loaded implants (NCT04527887, NCT04413279, NCT04658927) and hydrogels (NCT04541888, NCT04645446, NCT04884217), dietary supplements consisting of vitamins and lipids (NCT04181593) as well as physical activities for boosting well-being (NCT04421300).
Future prospects for DES treatment
As discussed, DES could be caused by a large variety of factors. However, current treatments mainly addressed the symptoms by hydrating or lubricating the ocular surface without tackling the root problems [148]. Besides creating unhealthy dependence in patients, such approaches will also lead to significant financial burden due to recurring treatment costs. Therefore, it is encouraging to witness the trajectory of upcoming DES treatment strategies where cellular and tissue regeneration in the LFU are the key focus. Specifically, studies that employ blood components such as platelet rich plasma or serum hold great promise in the clinics not only for treating DES but for other diseases as well [214]. However, like many other treatment options, allogeneic stem cell and body fluid therapy come with their own limitations that cannot be easily circumvented. Most notably, they involve invasive procedures and may deter patients from opting for this method. Additionally, the effectiveness of these components in inducing favorable outcomes is highly dependent on the patients’ suitability as well.
Therefore, for treatments to be inclusive, they should be varied and multipronged. In our opinion, one promising alternative is gene therapy, which enables the alteration of genetic sequences within tissues and cells with recombinant nucleic acids [215]. Commonly used nucleic acids such as DNA, mRNA, siRNA, miRNA and anti-sense oligonucleotides can be strategically delivered into a defective target cell or tissue in order to either restore the gene(s) responsible for disease suppression or inhibit the gene(s) related to disease development [216]. Besides its versatility, these nucleic acids can also be administered noninvasively for DES treatment. Accordingly, the efficacy of this technology will be investigated in one of the upcoming clinical trials listed in Table 2 which utilizes Tivanisiran (NCT04819269), a novel 19 nucleotide siRNA for suppressing the expression of the transient receptor potential cation channel subfamily V member 1 (TRPV1) [153]. TRPV1 is a pain receptor found in some components of the LFU [217] and the responses it mediates in the sensory neurons was found to be associated with the development of inflammation and neuropathic pain [218]. The delivery of this siRNA-based of eye drop will potentially result in the reduction in TRPV1’s expression in the ocular tissues and therefore, alleviate inflammation and improve tear secretion [219]. Of note, naked nucleic acids are very inefficiently uptaken by cells as they possess similar negative charges as the cell membrane, which leads to electrostatic repulsion [220, 221]. Delivery vehicles are required to transport nucleic acids across the cell membrane and herein determines the success of gene therapy. Recently, a breakthrough in vaccination strategy has shed valuable insights about the optimal form of nucleic acid carriers. Specifically, the Pfizer vaccine for Covid-19 utilizes a specially formulated liposome for delivering mRNAs into cells with great efficiency [222]. With the approval of this revolutionary delivery platform, gene therapy is thus in a favorable position to take off.
Another prospective DES treatment option is fecal microbiota transplantation (FMT), which is the transfer of fecal materials from a healthy donor into the intestinal tract of an ill or diseased recipient. By doing so, the recipient’s gut microbial composition can be adjusted to resemble the healthy donor’s, thereby conferring health benefits [223]. Since DES was found to be associated with gut dysbiosis, FMT is a potentially relevant and practical technique for treatment. However, there were not many clinical trials investigating the efficacy of FMT on DES patients as it was only quite recently that a correlation between DES and gut dysbiosis was uncovered. The first and only study was completed on June 2020, which explored the effects of FMT on patients with SS (NCT03926286). Alternatively, we may also expect ocular microbiota transplantation in future as studies have identified microbiome differences between closed dry eye patients and healthy closed eye patients [224,225,226].
Conclusion
DES is a relatively common ophthalmic disease that can manifest in various degrees of severity and can be caused by many factors. While not life threatening, patients may often have to continuously endure discomfort or even pain, which puts a damper in their quality of life. Given the multitude of conditions which DES can originate from, a variety of treatment options is critical to ensure inclusivity and effectiveness. Encouragingly, current clinical trials are trending towards this notion and investigating promising research-backed treatments like stem cell therapy, blood component therapy and gene therapy. If successful, these strategies may define treatments for other diseases in future as well.
Availability of data and material
Not applicable.
Code availability
Not applicable.
References
Moss SE, Klein R, Klein BEK (2000) Prevalence of and risk factors for dry eye syndrome. Arch Ophthalmol 9:1264–1268
(2007) The definition and classification of dry eye disease: report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf 2:75–92
Liu KC, Huynh K, Grubbs J Jr et al (2014) Autoimmunity in the pathogenesis and treatment of keratoconjunctivitis sicca. Curr Allergy Asthma Rep 1:403–403
Pflugfelder SC, Stern ME (2020) The cornea in keratoconjunctivitis sicca. Exp Eye Res. https://doi.org/10.1016/j.exer.2020.108295
Javadi MA, Feizi S (2011) Dry eye syndrome. J Ophthalmic Vis Res 3:192–198
Rolando M, Zierhut M (2001) The ocular surface and tear film and their dysfunction in dry eye disease. Surv Ophthalmol 45:S203–S210
King-Smith PE, Fink BA, Hill RM et al (2004) The thickness of the tear film. Curr Eye Res 4–5:357–368
King-Smith PE, Fink BA, Fogt N et al (2000) The thickness of the human precorneal tear film: evidence from reflection spectra. Invest Ophthalmol Vis Sci 11:3348–3359
Willcox MDP, Argüeso P, Georgiev GA et al (2017) TFOS DEWS II tear film report. Ocul Surf 3:366–403
King-Smith PE, Hinel EA, Nichols JJ (2010) Application of a novel interferometric method to investigate the relation between lipid layer thickness and tear film thinning. Invest Ophthalmol Vis Sci 5:2418–2423
Dilly PN (1994) Structure and function of the tear film. In: Sullivan DA (ed) Lacrimal gland, tear film, and dry eye syndromes: basic science and clinical relevance. Springer, Boston, pp 239–247
Cher I (2008) A new look at lubrication of the ocular surface: fluid mechanics behind the blinking eyelids. Ocul Surf 2:79–86
Peng CC, Cerretani C, Li Y et al (2014) Flow evaporimeter to assess evaporative resistance of human tear-film lipid layer. Ind Eng Chem Res 47:18130–18139
Bron AJ, Tiffany JM, Gouveia SM et al (2004) Functional aspects of the tear film lipid layer. Exp Eye Res 3:347–360
Knop E, Knop N, Millar T et al (2011) The international workshop on meibomian gland dysfunction: report of the subcommittee on anatomy, physiology, and pathophysiology of the meibomian gland. Invest Ophthalmol Vis Sci 4:1938–1978
Zhou L, Zhao SZ, Koh SK et al (2012) In-depth analysis of the human tear proteome. J Proteom 13:3877–3885
Azkargorta M, Soria J, Ojeda C et al (2015) Human basal tear peptidome characterization by CID, HCD, and ETD followed by in silico and in vitro analyses for antimicrobial peptide identification. J Proteome Res 6:2649–2658
Dartt DA, Willcox MDP (2013) Complexity of the tear film: importance in homeostasis and dysfunction during disease. Exp Eye Res 117:1–3
Mantelli F, Mauris J, Argüeso P (2013) The ocular surface epithelial barrier and other mechanisms of mucosal protection: from allergy to infectious diseases. Curr Opin Allergy Clin Immunol 5:563–568
Hattrup CL, Gendler SJ (2008) Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol 70:431–457
Thornton DJ, Rousseau K, McGuckin MA (2008) Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol 70:459–486
Gipson IK, Hori Y, Argüeso P (2004) Character of ocular surface mucins and their alteration in dry eye disease. Ocul Surf 2:131–148
Gipson IK, Argüeso P (2003) Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 231:1–49
Sumiyoshi M, Ricciuto J, Tisdale A et al (2008) Antiadhesive character of mucin O-glycans at the apical surface of corneal epithelial cells. Invest Ophthalmol Vis Sci 1:197–203
Lemp MA (1995) Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. Clao J 4:221–232
Johnson ME, Murphy PJ (2004) Changes in the tear film and ocular surface from dry eye syndrome. Prog Retin Eye Res 4:449–474
Stern ME, Schaumburg CS, Pflugfelder SC (2013) Dry eye as a mucosal autoimmune disease. Int Rev Immunol 1:19–41
Coursey TG, de Paiva CS (2014) Managing Sjögren’s Syndrome and non-Sjögren Syndrome dry eye with anti-inflammatory therapy. Clin Ophthalmol 8:1447–1458
Peck T, Olsakovsky L, Aggarwal S (2017) Dry eye syndrome in menopause and perimenopausal age group. J Mid-Life Health 2:51–54
Versura P, Giannaccare G, Campos EC (2015) Sex-steroid imbalance in females and dry eye. Curr Eye Res 2:162–175
Zhang X, Zhao L, Deng S et al (2016) Dry eye syndrome in patients with diabetes mellitus: prevalence, etiology, and clinical characteristics. J Ophthalmol 2016:8201053
De Freitas GR, Ferraz GAM, Gehlen M et al (2021) Dry eyes in patients with diabetes mellitus. Prim Care Diabetes 1:184–186
Abd-Allah NM, Hassan AA, Omar G et al (2020) Dry eye in rheumatoid arthritis: relation to disease activity. Immunol Med 2:92–97
Yumori JW, Trinh D, Lee E et al (2015) Prevalence of dry eye disease in rheumatoid arthritis patients. Invest Ophthalmol Vis Sci 7:4437–4437
Gold-von Simson G, Axelrod FB (2006) Familial dysautonomia: update and recent advances. Curr Probl Pediatr Adolesc Health Care 6:218–237
Mendoza-Santiesteban CE, Hedges TR 3rd, Norcliffe-Kaufmann L et al (2012) Clinical neuro-ophthalmic findings in familial dysautonomia. J Neuro-Ophthalmol 1:23–26
Galor A, Levitt RC, Felix ER et al (2015) Neuropathic ocular pain: an important yet underevaluated feature of dry eye. Eye (Lond) 3:301–312
Belmonte C, Nichols JJ, Cox SM et al (2017) TFOS DEWS II pain and sensation report. Ocul Surf 3:404–437
Moon J, Choi SH, Yoon CH et al (2020) Gut dysbiosis is prevailing in Sjögren’s syndrome and is related to dry eye severity. PLoS ONE 2:e0229029
Tsigalou C, Stavropoulou E, Bezirtzoglou E (2018) Current Insights in microbiome shifts in Sjogren’s syndrome and possible therapeutic interventions. Front Immunol 9:1106
Mandell JT, Idarraga M, Kumar N et al (2020) Impact of air pollution and weather on dry eye. J Clin Med 11:3740
Alves M, Novaes P, Morraye Mde A et al (2014) Is dry eye an environmental disease? Arq Bras Oftalmol 3:193–200
Xu L, Zhang W, Zhu X-Y et al (2016) Smoking and the risk of dry eye: a meta-analysis. Int J Ophthalmol 10:1480–1486
Erginturk Acar D, Acar U, Ozen Tunay Z et al (2017) The effects of smoking on dry eye parameters in healthy women. Cutan Ocul Toxicol 1:1–4
Khalil HE, Aboud S, Azzab M (2018) Comparative study between smokers and nonsmokers regarding dry eye. Delta J Ophthalmol 1:9–13
Akkaya S, Atakan T, Acikalin B et al (2018) Effects of long-term computer use on eye dryness. North Clin Istanbul 4:319–322
Koh S (2020) Contact lens wear and dry eye: beyond the known. Asia-Pac J Ophthalmol. https://doi.org/10.1097/APO.0000000000000329
Shtein RM (2011) Post-LASIK dry eye. Expert Rev Ophthalmol 5:575–582
Wong AHY, Cheung RKY, Kua WN et al (2019) Dry eyes after SMILE. Asia-Pac J Ophthalmol 5:397–405
Barr JY, Wang X, Meyerholz DK et al (2017) CD8 T cells contribute to lacrimal gland pathology in the nonobese diabetic mouse model of Sjögren syndrome. Immunol Cell Biol 8:684–694
Zhou D, Chen Y-T, Chen F et al (2012) Critical involvement of macrophage infiltration in the development of Sjögren’s syndrome-associated dry eye. Am J Pathol 3:753–760
Srivastava A, Makarenkova HP (2020) Innate immunity and biological therapies for the treatment of Sjögren’s syndrome. Int J Mol Sci 23:9172
Chen Y-T, Lazarev S, Bahrami AF et al (2012) Interleukin-1 receptor mediates the interplay between CD4+ T cells and ocular resident cells to promote keratinizing squamous metaplasia in Sjögren’s syndrome. Lab Investig 4:556–570
Chen FY, Lee A, Ge S et al (2017) Aire-deficient mice provide a model of corneal and lacrimal gland neuropathy in Sjögren’s syndrome. PLoS ONE 9:e0184916
Pflugfelder SC, Jones D, Ji Z et al (1999) Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren’s syndrome keratoconjunctivitis sicca. Curr Eye Res 3:201–211
Lam H, Bleiden L, de Paiva CS et al (2009) Tear cytokine profiles in dysfunctional tear syndrome. Am J Ophthalmol 2:198–205
Solomon A, Dursun D, Liu Z et al (2001) Pro- and anti-inflammatory forms of interleukin-1 in the tear fluid and conjunctiva of patients with dry-eye disease. Invest Ophthalmol Vis Sci 10:2283–2292
Kook KY, Jin R, Li L et al (2020) Tear osmolarity and matrix metallopeptidase-9 in dry eye associated with Sjögren’s syndrome. Korean J Ophthalmol 3:179–186
Lanza NL, Valenzuela F, Perez VL et al (2016) The matrix metalloproteinase 9 point-of-care test in dry eye. Ocul Surf 2:189–195
Tsubota K, Fukagawa K, Fujihara T et al (1999) Regulation of human leukocyte antigen expression in human conjunctival epithelium. Invest Ophthalmol Vis Sci 1:28–34
Brignole-Baudouin F, Riancho L, Ismail D et al (2017) Correlation between the inflammatory marker HLA-DR and signs and symptoms in moderate to severe dry eye disease. Invest Ophthalmol Vis Sci 4:2438–2448
Akpek EK, Wu HY, Karakus S et al (2020) Differential diagnosis of sjögren versus non-sjögren dry eye through tear film biomarkers. Cornea 8:991–997
Gürdal C, Saraç O, Genç I et al (2011) Ocular surface and dry eye in Graves’ disease. Curr Eye Res 1:8–13
Bothun ED, Scheurer RA, Harrison AR et al (2009) Update on thyroid eye disease and management. Clin Ophthalmol 3:543–551
Iskeleli G, Karakoc Y, Abdula A (2008) Tear film osmolarity in patients with thyroid ophthalmopathy. Jpn J Ophthalmol 4:323–326
Eckstein AK, Finkenrath A, Heiligenhaus A et al (2004) Dry eye syndrome in thyroid-associated ophthalmopathy: lacrimal expression of TSH receptor suggests involvement of TSHR-specific autoantibodies. Acta Ophthalmol Scand 3(Pt 1):291–297
Coyle PK, Sibony PA (1986) Tear analysis in multiple sclerosis. Neurology 4:547–550
Pontelli RCN, Rocha BA, Garcia DM et al (2020) Endocrine disrupting chemicals associated with dry eye syndrome. Ocul Surf 3:487–493
Sullivan DA, Rocha EM, Aragona P et al (2017) TFOS DEWS II sex, gender, and hormones report. Ocul Surf 3:284–333
Richards SM, Jensen RV, Liu M et al (2006) Influence of sex on gene expression in the mouse lacrimal gland. Exp Eye Res 1:13–23
Suzuki T, Schirra F, Richards SM et al (2006) Estrogen’s and progesterone’s impact on gene expression in the mouse lacrimal gland. Invest Ophthalmol Vis Sci 1:158–168
Toda I, Wickham LA, Sullivan DA (1998) Gender and androgen treatment influence the expression of proto-oncogenes and apoptotic factors in lacrimal and salivary tissues of MRL/lprMice. Clin Immunol Immunopathol 1:59–71
Richards SM, Liu M, Sullivan BD et al (2002) Gender-related differences in gene expression of the lacrimal gland. In: Sullivan DA, Stern ME, Tsubota K, Dartt DA, Sullivan RM, Bromberg BB (eds) Lacrimal gland, tear film, and dry eye syndromes 3: basic science and clinical relevance part A and B. Springer, Boston, pp 121–127
Gao J, Lambert RW, Wickham LA et al (1995) Androgen control of secretory component mRNA levels in the rat lacrimal gland. J Steroid Biochem Mol Biol 3:239–249
Ono M, Rocha FJ, Sullivan DA (1995) Immunocytochemical location and hormonal control of androgen receptors in lacrimal tissues of the female MRL/Mp-Ipr/Ipr mouse model of sjögren’s syndrome. Exp Eye Res 6:659–666
Truong S, Cole N, Stapleton F et al (2014) Sex hormones and the dry eye. Clin Exp Optom 4:324–336
Chia EM, Mitchell P, Rochtchina E et al (2003) Prevalence and associations of dry eye syndrome in an older population: the Blue Mountains Eye Study. Clin Exp Ophthalmol 3:229–232
Sullivan DA, Wickham LA, Rocha EM et al (1998) Influence of gender, sex steroid hormones, and the hypothalamic-pituitary axis on the structure and function of the lacrimal gland. In: Sullivan DA, Dartt DA, Meneray MA (eds) Lacrimal gland, tear film, and dry eye syndromes 2: basic science and clinical relevance. Springer, Boston, pp 11–42
Carlsen SM, Jacobsen G, Vanky E (2010) Mid-pregnancy androgen levels are negatively associated with breastfeeding. Acta Obstet Gynecol Scand 1:87–94
Yasui T, Matsui S, Tani A et al (2012) Androgen in postmenopausal women. J Med Invest 1–2:12–27
Thody AJ, Shuster S (1989) Control and function of sebaceous glands. Physiol Rev 2:383–416
Sullivan DA, Sullivan BD, Evans JE et al (2002) Androgen deficiency, Meibomian gland dysfunction, and evaporative dry eye. Ann N Y Acad Sci 966:211–222
Schröder HG, Ziegler M, Nickisch K et al (1989) Effects of topically applied antiandrogenic compounds on sebaceous glands of hamster ears and flank organs. J Invest Dermatol 5:769–773
Manaviat MR, Rashidi M, Afkhami-Ardekani M et al (2008) Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol 8:10
Zou X, Lu L, Xu Y et al (2018) Prevalence and clinical characteristics of dry eye disease in community-based type 2 diabetic patients: the Beixinjing eye study. BMC Ophthalmol 1:117
Najafi L, Malek M, Valojerdi AE et al (2013) Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Complicat 5:459–462
Gekka M, Miyata K, Nagai Y et al (2004) Corneal epithelial barrier function in diabetic patients. Cornea 1:35–37
Nitin S (2010) HbA1c and factors other than diabetes mellitus affecting it. Singapore Med J 8:616–622
Tang WH, Martin KA, Hwa J (2012) Aldose reductase, oxidative stress, and diabetic mellitus. Front Pharmacol 3:87
Faustino JF, Ribeiro-Silva A, Dalto RF et al (2016) Vitamin A and the eye: an old tale for modern times. Arquivos Bras Oftalmol 79:56–61
Pal R, Sagar V (2008) Antecedent risk factors of xerophthalmia among Indian rural preschool children. Eye Contact Lens 2:106–108
McLaughlin S, Welch J, MacDonald E et al (2014) Xerophthalmia: a potential epidemic on our doorstep? Eye (Lond) 5:621–623
Feroze KB, Kaufman EJ (2021) Xerophthalmia. StatPearls Publishing, Copyright © 2021, StatPearls Publishing LLC, Treasure Island
Zhang W, Li W, Zhang C et al (2019) Effects of vitamin A on Expressions Of Apoptosis Genes Bax and Bcl-2 in epithelial cells of corneal tissues induced by benzalkonium chloride in mice with dry eye. Med Sci Monit 25:4583–4589
Palma J-A, Norcliffe-Kaufmann L, Fuente-Mora C et al (2014) Current treatments in familial dysautonomia. Expert Opin Pharmacother 18:2653–2671
Nikkinen A, Lehtosalo JI, Uusitalo H et al (1984) The lacrimal glands of the rat and the guinea pig are innervated by nerve fibers containing immunoreactivities for substance P and vasoactive intestinal polypeptide. Histochemistry 1:23–27
Seifert P, Spitznas M (1999) Vasoactive intestinal polypeptide (VIP) innervation of the human eyelid glands. Exp Eye Res 6:685–692
Matsumoto Y, Tanabe T, Ueda S et al (1992) Immunohistochemical and enzymehistochemical studies of peptidergic, aminergic and cholinergic innervation of the lacrimal gland of the monkey (Macaca fuscata). J Auton Nerv Syst 3:207–214
Marty A, Tan YP, Trautmann A (1984) Three types of calcium-dependent channel in rat lacrimal glands. J Physiol 357:293–325
Trautmann A, Marty A (1984) Activation of Ca-dependent K channels by carbamoylcholine in rat lacrimal glands. Proc Natl Acad Sci U S A 2:611–615
Wood RL, Mircheff AK (1986) Apical and basal-lateral Na/K-ATPase in rat lacrimal gland acinar cells. Invest Ophthalmol Vis Sci 8:1293–1296
Putney JW Jr, VandeWalle CM, Leslie BA (1978) Stimulus-secretion coupling in the rat lacrimal gland. Am J Physiol 5:C188-198
Seifert P, Spitznas M (1996) Immunocytochemical and ultrastructural evaluation of the distribution of nervous tissue and neuropeptides in the meibomian gland. Graefes Arch Clin Exp Ophthalmol 10:648–656
Diebold Y, Ríos JD, Hodges RR et al (2001) Presence of nerves and their receptors in mouse and human conjunctival goblet cells. Invest Ophthalmol Vis Sci 10:2270–2282
Kirch W, Horneber M, Tamm ER (1996) Characterization of Meibomian gland innervation in the cynomolgus monkey (Macaca fascicularis). Anat Embryol (Berl) 4:365–375
Andersen HH, Yosipovitch G, Galor A (2017) Neuropathic symptoms of the ocular surface: dryness, pain, and itch. Curr Opin Allergy Clin Immunol 5:373–381
Staaf S, Oerther S, Lucas G et al (2009) Differential regulation of TRP channels in a rat model of neuropathic pain. PAIN® 1:187–199
Habib AM, Wood JN, Cox JJ (2015) Sodium channels and pain. Handbook of experimental pharmacology. Springer, Berlin, pp 39–56
Tibbs GR, Posson DJ, Goldstein PA (2016) Voltage-gated ion channels in the PNS: novel therapies for neuropathic pain? Trends Pharmacol Sci 7:522–542
Lloyd-Price J, Abu-Ali G, Huttenhower C (2016) The healthy human microbiome. Genome Med 1:51
Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 8:e1002533
Kho ZY, Lal SK (2018) The human gut microbiome: a potential controller of wellness and disease. Front Microbiol. https://doi.org/10.3389/fmicb.2018.01835
Hooper LV, Gordon JI (2001) Commensal host-bacterial relationships in the gut. Science 5519:1115
Moon J, Yoon CH, Choi SH et al (2020) Can gut microbiota affect dry eye syndrome? Int J Mol Sci 22:8433
Trujillo-Vargas CM, Schaefer L, Alam J et al (2020) The gut-eye-lacrimal gland-microbiome axis in Sjögren Syndrome. Ocul Surf 2:335–344
Saxena R, Srivastava S, Trivedi D et al (2003) Impact of environmental pollution on the eye. Acta Ophthalmol Scand 5:491–494
Wiwatanadate P (2014) Acute air pollution-related symptoms among residents in Chiang Mai, Thailand. J Environ Health 6:76–84
Kiotseridis H, Cilio CM, Bjermer L et al (2013) Grass pollen allergy in children and adolescents-symptoms, health related quality of life and the value of pollen prognosis. Clin Transl Allergy 3:1–19
Idarraga MA, Guerrero JS, Mosle SG et al (2020) Relationships between short-term exposure to an indoor environment and dry eye (DE) symptoms. J Clin Med 5:1316
Rios JL, Boechat JL, Gioda A et al (2009) Symptoms prevalence among office workers of a sealed versus a non-sealed building: associations to indoor air quality. Environ Int 8:1136–1141
Lu C, Deng Q, Li Y et al (2016) Outdoor air pollution, meteorological conditions and indoor factors in dwellings in relation to sick building syndrome (SBS) among adults in China. Sci Total Environ 560–561:186–196
Jung SJ, Mehta JS, Tong L (2018) Effects of environment pollution on the ocular surface. Ocul Surf 2:198–205
Zhong JY, Lee YC, Hsieh CJ et al (2018) Association between dry eye disease, air pollution and weather changes in Taiwan. Int J Environ Res Public Health 10:2269
Mo Z, Fu Q, Lyu D et al (2019) Impacts of air pollution on dry eye disease among residents in Hangzhou, China: a case-crossover study. Environ Pollut 246:183–189
Hwang SH, Choi YH, Paik HJ et al (2016) Potential importance of ozone in the association between outdoor air pollution and dry eye disease in South Korea. JAMA Ophthalmol 5:503–510
Um SB, Kim NH, Lee HK et al (2014) Spatial epidemiology of dry eye disease: findings from South Korea. Int J Health Geogr 13:31
van Setten G, Labetoulle M, Baudouin C et al (2016) Evidence of seasonality and effects of psychrometry in dry eye disease. Acta Ophthalmol 5:499–506
Lam DCL, Nana A, Eastwood PR et al (2014) Electronic cigarettes: ‘vaping’ has unproven benefits and potential harm. Respirology 7:945–947
Md Isa NA, Koh PY, Doraj P (2019) The tear function in electronic cigarette smokers. Optom Vis Sci 9:678–685
Thomas J, Jacob GP, Abraham L et al (2012) The effect of smoking on the ocular surface and the precorneal tear film. Australas Med J 4:221–226
Randolph SA (2017) Computer vision syndrome. Workplace Health Saf 7:328
Stapleton F, Keay L, Jalbert I et al (2007) The epidemiology of contact lens related infiltrates. Optom Vis Sci 4:257–272
Markoulli M, Kolanu S (2017) Contact lens wear and dry eyes: challenges and solutions. Clin Optom 9:41–48
Riley C, Young G, Chalmers R (2006) Prevalence of ocular surface symptoms, signs, and uncomfortable hours of wear in contact lens wearers: the effect of refitting with daily-wear silicone hydrogel lenses (senofilcon a). Eye Contact Lens 6:281–286
Doughty MJ (2011) Contact lens wear and the goblet cells of the human conjunctiva: a review. Cont Lens Anterior Eye 4:157–163
Colorado LH, Alzahrani Y, Pritchard N et al (2016) Time course of changes in goblet cell density in symptomatic and asymptomatic contact lens wearers. Invest Ophthalmol Vis Sci 6:2888–2894
Sapkota K, Franco S, Sampaio P et al (2016) Effect of three months of soft contact lens wear on conjunctival cytology. Clin Exp Optom 4:336–341
Nichols JJ, Mitchell GL, King-Smith PE (2005) Thinning rate of the precorneal and prelens tear films. Invest Ophthalmol Vis Sci 7:2353–2361
Sindt CW, Longmuir RA (2007) Contact lens strategies for the patient with dry eye. Ocul Surf 4:294–307
Bower KS, Sia RK, Ryan DS et al (2015) Chronic dry eye in photorefractive keratectomy and laser in situ keratomileusis: manifestations, incidence, and predictive factors. J Cataract Refract Surg 12:2624–2634
Ambrósio R Jr, Tervo T, Wilson SE (2008) LASIK-associated dry eye and neurotrophic epitheliopathy: pathophysiology and strategies for prevention and treatment. J Refract Surg 4:396–407
Toda I (2018) Dry eye after LASIK. Invest Ophthalmol Vis Sci 14:DES109–DES115
Ang RT, Dartt DA, Tsubota K (2001) Dry eye after refractive surgery. Curr Opin Ophthalmol 4:318–322
Battat L, Macri A, Dursun D et al (2001) Effects of laser in situ keratomileusis on tear production, clearance, and the ocular surface. Ophthalmology 7:1230–1235
Rodriguez AE, Rodriguez-Prats JL, Hamdi IM et al (2007) Comparison of goblet cell density after femtosecond laser and mechanical microkeratome in LASIK. Invest Ophthalmol Vis Sci 6:2570–2575
Konomi K, Chen LL, Tarko RS et al (2008) Preoperative characteristics and a potential mechanism of chronic dry eye after LASIK. Invest Ophthalmol Vis Sci 1:168–174
Mian SI, Li AY, Dutta S et al (2009) Dry eyes and corneal sensation after laser in situ keratomileusis with femtosecond laser flap creation Effect of hinge position, hinge angle, and flap thickness. J Cataract Refract Surg 12:2092–2098
O’Neil EC, Henderson M, Massaro-Giordano M et al (2019) Advances in dry eye disease treatment. Curr Opin Ophthalmol 3:166–178
Yeu E, Goldberg DF, Mah FS et al (2019) Safety and efficacy of amniotic cytokine extract in the treatment of dry eye disease. Clin Ophthalmol 13:887–894
Iorio R, Petricca S, Luzi C et al (2021) Lactobacillus sakei Pro-Bio65 reduces TNF-α expression and upregulates GSH content and antioxidant enzymatic activities in human conjunctival cells. Transl Vis Sci Technol 6:8–8
Alio JL, Rodriguez AE, Ferreira-Oliveira R et al (2017) Treatment of dry eye disease with autologous platelet-rich plasma: a prospective, interventional, non-randomized study. Ophthalmol Ther 2:285–293
You J, Hodge C, Hoque M et al (2020) Human platelets and derived products in treating ocular surface diseases: a systematic review. Clin Ophthalmol 14:3195–3210
Gonzalez V, Ruz V, Bleau AM et al (2020) Tivanisiran as a new treatment for Dry Eye in patients with Sjögren Syndrome. Invest Ophthalmol Vis Sci 7:102–102
Jimenez AI, Ruz V, Gonzalez V et al (2018) Tivanisiran, a new treatment for Dry Eye Disease, that improved signs and symptoms in clinical trials. Invest Ophthalmol Vis Sci 9:925–925
Johnson ME, Murphy PJ, Boulton M (2006) Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch Clin Exp Ophthalmol 1:109–112
Ang BCH, Sng JJ, Wang PXH et al (2017) Sodium hyaluronate in the treatment of dry eye syndrome: a systematic review and meta-analysis. Sci Rep 1:9013–9013
Garrett Q, Zhang X, Wang Y et al (2013) Topical administration of L-carnitine on prevention and treatment of murine dry eye. Invest Ophthalmol Vis Sci 15:921–921
Nam K, Kim HJ, Yoo A (2019) Efficacy and safety of topical 3% diquafosol ophthalmic solution for the treatment of multifactorial dry eye disease: meta-analysis of randomized clinical Trials. Ophthalmic Res 4:188–198
Watanabe H (2018) Medical treatment for dry eye in Japan. Investig Ophthalmol Vis Sci 14:DES116–DES120
Wirta DL, Torkildsen GL, Moreira HR et al (2019) A clinical phase ii study to assess efficacy, safety, and tolerability of waterfree cyclosporine formulation for treatment of dry eye disease. Ophthalmology 6:792–800
Yang L, Yang Z, Yu H et al (2015) Acupuncture therapy is more effective than artificial tears for dry eye syndrome: evidence based on a meta-analysis. Evid-Based Complement Altern Med 2015:1–11
Borchman D (2019) The optimum temperature for the heat therapy for meibomian gland dysfunction. Ocul Surf 2:360–364
Lallemand F, Schmitt M, Bourges J-L et al (2017) Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur J Pharm Biopharm 117:14–28
Sheha H, Tighe S, Hashem O et al (2019) Update on cenegermin eye drops in the treatment of neurotrophic keratitis. Clin Ophthalmol 13:1973–1980
Shin M, Ahn H, Bernton E et al (2019) HL036 ophthalmic solution, a topical TNF-α inhibitor, significantly improves signs and symptoms of dry eye in a phase 2 clinical trial (VELOS-1). Invest Ophthalmol Vis Sci 9:249–249
Crawford KS, Schuh C, Schuh J et al (2019) Effects of ALY688 on atropine-induced dry eye in rabbits. Invest Ophthalmol Vis Sci 9:305–305
Usuba FS, de Medeiros-Ribeiro AC, Novaes P et al (2020) Dry eye in rheumatoid arthritis patients under TNF-inhibitors: conjunctival goblet cell as an early ocular biomarker. Sci Rep 1:14054
Ji YW, Byun YJ, Choi W et al (2013) Neutralization of ocular surface TNF-α reduces ocular surface and lacrimal gland inflammation induced by in vivo dry eye. Invest Ophthalmol Vis Sci 12:7557–7566
Villatoro AJ, Fernández V, Claros S et al (2015) Use of adipose-derived mesenchymal stem cells in keratoconjunctivitis sicca in a canine model. BioMed Res Int 2015:527926
Aniah Azmi N, Bastion MC (2020) Short-term results of trial of topical insulin for treatment of dry eyes in diabetics. Eye Contact Lens 46:S25–S32
Alio JL, Colecha JR, Pastor S et al (2007) Symptomatic dry eye treatment with autologous platelet-rich plasma. Ophthalmic Res 3:124–129
López-Plandolit S, Morales MC, Freire V et al (2011) Efficacy of plasma rich in growth factors for the treatment of dry eye. Cornea 12:1312–1317
Geerling G, MacLennan S, Hartwig D (2004) Autologous serum eye drops for ocular surface disorders. Br J Ophthalmol 11:1467
Yoon KC (2014) Use of umbilical cord serum in ophthalmology. Chonnam Med J 3:82–85
Giannaccare G, Carnevali A, Senni C et al (2020) umbilical cord blood and serum for thetreatment of ocular diseases: a comprehensive review. Ophthalmol Therapy 2:235–248
Kagkelaris KA, Makri OE, Georgakopoulos CD et al (2018) An eye for azithromycin: review of the literature. Ther Adv Ophthalmol 10:2515841418783622
(2019) Cyclosporine 0.09% solution (Cequa) for dry eye Disease. Med Lett Drugs Ther 1577:116–118
Friedlaender MH (1998) Twice-a-day versus four-times-a-day ofloxacin treatment of external ocular infection. Clao J 1:48–51
Tan J, Liu H, Huang M et al (2020) Small molecules targeting RORγt inhibit autoimmune disease by suppressing Th17 cell differentiation. Cell Death Dis 8:697
Clark D, Tauber J, Sheppard J et al (2021) Early onset and broad activity of reproxalap in a randomized, double-masked, vehicle-controlled phase 2b trial in dry eye disease. Am J Ophthalmol 226:22–31
Macdonald S, Halilovic A, Brady T (2018) Novel small molecule aldehyde sequestering agents demonstrate broad therapeutic potential for ocular inflammation. Invest Ophthalmol Vis Sci 9:2663–2663
McDonald MB, Sheha H, Tighe S et al (2018) Treatment outcomes in the DRy Eye Amniotic Membrane (DREAM) study. Clin Ophthalmol 12:677–681
Cai M-M, Zhang J (2020) Effectiveness of transcutaneous electrical stimulation combined with artificial tears for the treatment of dry eye: a randomized controlled trial. Exp Ther Med 6:175–175
Badawi D (2018) A novel system, TearCare(®), for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol 12:683–694
Ruggeri A, Fatigati E, Vigo L (2020) Mixed dry eye patients successfully treated by the innovative high-frequency electrotherapy device Rexon-Eye®. Invest Ophthalmol Vis Sci 7:114–114
Connor CG, Narayanan S, Miller WL (2016) The efficacy of MiBo Thermoflo in treatment of meibomian gland dysfunction. Invest Ophthalmol Vis Sci 12:5678–5678
Ji MH, Moshfeghi DM, Periman L et al (2020) Novel extranasal tear stimulation: pivotal study results. Transl Vis Sci Technol 12:23–23
Keir N, Woods CA, Dumbleton K et al (2010) Clinical performance of different care systems with silicone hydrogel contact lenses. Cont Lens Anterior Eye 4:189–195
Lazon de la Jara P, Papas E, Diec J et al (2013) Effect of lens care systems on the clinical performance of a contact lens. Optom Vis Sci 4:344–350
Lee A, Blair HA (2020) Dexamethasone intracanalicular insert: a review in treating post-surgical ocular pain and inflammation. Drugs 11:1101–1108
Luo ZK, Domenech-Estarellas E, Han A et al (2020) A novel transdermal treatment of chronic ocular graft-vs-host disease (oGvHD): a phase II Clinic trial. Biol Blood Marrow Transplant 3(Supplement):S31–S32
Li J, Ruzhi D, Hua X et al (2016) Blueberry component pterostilbene protects corneal epithelial cells from inflammation via anti-oxidative pathway. Sci Rep 6:19408
Marquardt R (1986) Treatment of dry eye with a new gel in eyedrop form. Klin Monbl Augenheilkd 1:51–54
Greiner JV (2012) A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res 4:272–278
Bains KK, Fukuoka H, Hammond GM et al (2019) Recovering vision in corneal epithelial stem cell deficient eyes. Cont Lens Anterior Eye 4:350–358
Nurković JS, Vojinović R, Dolićanin Z (2020) Corneal stem cells as a source of regenerative cell-based therapy. Stem Cells Int 2020:1–11
Yeo S, Tong L (2018) Coping with dry eyes: a qualitative approach. BMC Ophthalmol 1:8
Tauber J, Owen J, Bloomenstein M et al (2020) Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol 14:405–418
Belalcázar-Rey S, Sánchez Huerta V, Ochoa-Tabares JC et al (2020) Efficacy and safety of sodium hyaluronate/chondroitin sulfate preservative-free ophthalmic solution in the treatment of dry eye: a clinical trial. Curr Eye Res 46:919–929
Sosne G, Qiu P, Ousler GW et al (2012) Thymosin β4: a potential novel dry eye therapy. Ann N Y Acad Sci 1270:45–50
Sosne G, Kleinman HK (2015) Primary mechanisms of thymosin β4 repair activity in dry eye disorders and other tissue injuries. Invest Ophthalmol Vis Sci 9:5110–5117
Postorino EI, Rania L, Aragona E et al (2018) Efficacy of eyedrops containing cross-linked hyaluronic acid and coenzyme Q10 in treating patients with mild to moderate dry eye. Eur J Ophthalmol 1:25–31
Song JK, Lee K, Park HY et al (2017) Efficacy of carboxymethylcellulose and hyaluronate in dry eye disease: a systematic review and meta-analysis. Korean J Fam Med 1:2–7
Toyos R, Toyos M, Willcox J et al (2019) Evaluation of the safety and efficacy of intense pulsed light treatment with meibomian gland expression of the upper eyelids for dry eye disease. Photobiomodul Photomed Laser Surg 9:527–531
Ferreira FC, Sathler CSCdO, Hida IY et al (2020) Upper eyelid blepharoplasty using plasma exeresis: evaluation of outcomes, satisfaction, and symptoms after procedure. J Cosmet Dermatol 20:2758–2764
Park J, Yoo Y-S, Shin E et al (2020) Effects of the re-esterified triglyceride (rTG) form of omega-3 supplements on dry eye following cataract surgery. Br J Ophthalmol 105:1504–1509
Kymionis GD, Bouzoukis DI, Diakonis VF et al (2008) Treatment of chronic dry eye: focus on cyclosporine. Clin Ophthalmol 4:829–836
Schultz C (2014) Safety and efficacy of cyclosporine in the treatment of chronic dry eye. Ophthalmol Eye Dis 6:37–42
Mori S, Naito M, Moriyama S (2002) Highly viscous sodium hyaluronate and joint lubrication. Int Orthop 2:116–121
Romanò CL, De Vecchi E, Bortolin M et al (2017) Hyaluronic acid and its composites as a local antimicrobial/antiadhesive barrier. J Bone Joint Infect 1:63–72
Kummara MR, Kumar A, Sung Soo H (2017) Development of antibacterial paper coated with sodium hyaluronate stabilized curcumin-Ag nanohybrid and chitosan via polyelectrolyte complexation for medical applications. Mater Res Express 11:115401
Arita R, Fukuoka S (2020) Non-pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom 6:742–755
Sella S, Adami V, Amati E et al (2018) In-vitro analysis of quantum molecular resonance effects on human mesenchymal stromal cells. PLoS ONE 1:e0190082
Kabat M, Bobkov I, Kumar S et al (2020) Trends in mesenchymal stem cell clinical trials 2004–2018: is efficacy optimal in a narrow dose range? Stem Cells Transl Med 1:17–27
Hanna E, Rémuzat C, Auquier P et al (2017) Gene therapies development: slow progress and promising prospect. J Mark Access Health Policy 1:1265293–1265293
Goswami R, Subramanian G, Silayeva L et al (2019) Gene therapy leaves a vicious cycle. Front Oncol. https://doi.org/10.3389/fonc.2019.00297
Yang JM, Wei ET, Kim SJ et al (2018) TRPM8 channels and dry eye. Pharmaceuticals 4:125
Fischer MJM, Btesh J, McNaughton PA (2013) Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J Neurosci 17:7407
Masuoka T, Yamashita Y, Nakano K et al (2020) Chronic tear deficiency sensitizes transient receptor potential vanilloid 1-mediated responses in corneal sensory nerves. Front Cell Neurosci. https://doi.org/10.3389/fncel.2020.598678
Huo S, Li H, Boersma AJ et al (2019) DNA nanotechnology enters cell membranes. Adv Sci 10:1900043–1900043
Lin J, Jo SB, Kim T-H et al (2020) RNA interference in glial cells for nerve injury treatment. J Tissue Eng 11:2041731420939224–2041731420939224
(2021) Let’s talk about lipid nanoparticles. Nat Rev Mater 2:99–99
Gupta S, Allen-Vercoe E, Petrof EO (2016) Fecal microbiota transplantation: in perspective. Ther Adv Gastroenterol 2:229–239
Willis KA, Postnikoff CK, Freeman AB et al (2020) The closed eye harbors a unique microbiome in dry eye disease. medRxiv 182:90
Gomes JÁP, Frizon L, Demeda VF (2020) Ocular surface microbiome in health and disease. Asia-Pac J Ophthalmol 6:505–511
Li JJ, Yi S, Wei L (2020) Ocular microbiota and intraocular inflammation. Front Immunol. https://doi.org/10.3389/fimmu.2020.609765
Author information
Authors and Affiliations
Contributions
RJH, CYS, LJF and JQL wrote and edited the manuscript. JSC and YD proofread and edited the manuscript. JSC and YD provided guidance and the funding for this work.
Corresponding authors
Ethics declarations
Conflict of interest
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, R., Su, C., Fang, L. et al. Dry eye syndrome: comprehensive etiologies and recent clinical trials. Int Ophthalmol 42, 3253–3272 (2022). https://doi.org/10.1007/s10792-022-02320-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-022-02320-7