Abstract
N-Acetylcysteine (NAC) is a chemical compound with anti-inflammatory and antioxidant activity and acts as a free radical scavenger. Elaeagnus angustifolia (EA) is a plant native to the western part of Iran, with antioxidant and anti-inflammatory properties. The present study been taken evaluated the protective effect afforded by EA and NAC extracts on carrageenan-induced acute lung injury in Wistar rats. In this study, 42 rats were randomly assigned into seven groups. NAC and EA extracts were orally administered once/day for 21 continuous days. Pulmonary damage was induced by intratracheal injection of 100 μl of 2% λ-Carrageenan on day 21. Twenty-four hours post-surgery, the rats were euthanized and the samples were collected. Pretreatment with NAC and EA extracts reduced the total and differential cell accumulation as well as IL-6, and TNF-α cytokines. Antioxidant indicators demonstrate that in the groups receiving NAC and EA extract, MDA decreased while thiol and antioxidant capacity elevated. Treatment with NAC and EA significantly reduced Carrageenan-induced pathological pulmonary tissue injury. NAC and EA extract has protective effects on acute carrageenan-induced lung injury.
Similar content being viewed by others
Introduction
Acute lung injury (ALI) and it’s more severe form, Acute respiratory distress syndrome (ARDS), are clinical complications with high mortality, increased permeability of the alveolar-capillary barrier impairs the function of pulmonary capillary endothelial cells, (Wu et al. 2020; Gurusamy et al. 2021) and contributes to high protein content and pulmonary edema (Laffey and Matthay 2017). ALI can result from infection, trauma, burns, or sepsis. Pro-inflammatory mediators released following tissue damage can lead to tissue damage, respiratory failure, and subsequent infiltration of neutrophil and other inflammatory cells into the interstitial and alveolar spaces. Activation and transport of neutrophils are important in developing ALI (Lee et al. 2019). Neutrophils play an essential role in the innate immune system, as these cells are the first leukocytes to migrate to areas of acute inflammation (Bian et al. 2012). Neutrophils secrete large amounts of cytokines and chemokines that affect other immune cells and are, therefore, the primary regulators of inflammation (Hilda and Das 2016). About 5% of patients with coronavirus disease 2019 (COVID-19) suffer from exacerbations of ARDS, septic shock, or multiple organ failure, and require hospitalization in the intensive care unit (ICU) (De Flora et al. 2020). ARDS is the leading cause of death in COVID-19 patients, including cytokine storm and systemic inflammatory response caused by the release of many pro-inflammatory cytokines and chemokines that trigger the immune system to attack (Li et al. 2020). High levels of pro-inflammatory cytokines may cause extensive lung damage, leading to widespread infiltration of neutrophils and macrophages, impaired alveolar diffusion by forming hyaline membranes, and diffuse thickening of the alveolar wall (Cao 2020). Inflammation is treated with nonsteroidal anti-inflammatory drugs (NSAIDs) or steroidal anti-inflammatory-immunity drugs (SAIDs). NSAIDs cause serious gastrointestinal injury and hepatotoxicity. SAIDs may result in sodium retention, obesity, and osteoporosis; these result in serious health problems (Ou et al. 2019). Besides, drugs and methods currently used to treat acute lung injury have not responded to patients fully. Despite decades of efforts to treat acute lung injury, the mortality rate of patients with an acute injury is still higher than 50% (Ye and Liu 2020). N-acetylcysteine) NAC (, is a chemical compound that controls and reduces inflammatory mediators (Bhatia and Moochhala 2004). NAC can directly scavenge oxygen free radicals and has immune regulatory effects such as inhibiting tumor necrosis factor-ɑ(TNF-ɑ) overproduction, increasing T cell activity, and improving the oxidation state, especially under severe oxidative stress (Qiao et al. 2019; Shen et al. 2016). Elaeagnus Angustifolia (EA) is one of the herbal medicines traditionally used to treat rheumatoid arthritis, osteoporosis, and asthma due in part to its therapeutic effects, such as antioxidant, anti-inflammatory, and antimicrobial, activities (Tehranizadeh et al. 2016). Phytochemical studies of EA fruit extract have identified various chemicals as flavonoids, phytosterols, polar glycosides, terpenoids, coumarins, phenols, carboxylic acids, saponins, and carotenoids (Okmen and Turkcan 2014). To the best of our knowledge, there is no study on its effect on pulmonary inflammation. Carrageenan is a potent inflammatory agent with neutrophil induction capacity and the production of reactive oxygen species (ROS). Induction of inflammation by carrageenan elevates the levels of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 (Alam et al. 2019, de Campos Facchin et al. 2020). Accordingly, the present study aimed to elucidate the anti-inflammatory effect of NAC and EA on Carrageenan-induced acute lung injury in mice.
Materials and methods
Materials
λ-Carrageenan (EC Number 232–953-5) was purchased from (Sigma Co. Steinheim, Germany.) N-acetylcysteine (NAC) tablets from (HEXAL Co. Holzkirchen, Germany) were used.
Preparation of aqueous extract of EA fruit
To prepare the aqueous extract of Elaeagnus angustifolia fruit (herbarium code E-1180 FUMH), 5 g of EA powder was mixed with 100 ml of distilled water and boiled at 95 °C for 15 min. The mixture was centrifuged at 2000 rpm for 3 min. Finally, the extract was filtered using Whatman filter paper, stored at −30 °C for 24 h, and then dried using the freeze-drying technique.
Experimental animals
42 male Wistar rats (200–250 g) were enrolled in this research. All rats were kept under standard conditions, 22 ± 2 °C, 12 h of illumination, and 12 h of darkness. During the experiment, rats had free access to water and food. All experiments with laboratory animals were performed according to the instructions of the ethics committee of Birjand University of Medical Sciences (Birjand, Iran). The experiments were reviewed and approved, with ethics code IR.BUMS.REC.1399.474.
Animal groups
Animals were randomly assigned into 7 groups of Control (saline), Carrageenan (intratracheal instillation of 0.1 ml, 2%), Carrageenan + EA (100 mg/kg, gavage), Carrageenan + EA (400 mg/kg, gavage), Carrageenan + NAC (50 mg/kg, gavage), Carrageenan + NAC (200 mg/kg, gavage) and Carrageenan + EA + NAC (400 mg/kg, gavage).
Carrageenan-induced acute lung injury
Rats were treated with EA extract and NAC for 21 days. On day 22, mice were anesthetized with ketamine [100 mg/kg, intramuscular (i.m)] and xylazine (10 mg/kg, i.m). ALI was induced by 0.1 ml intratracheal injection of 2% carrageenan in saline. Rats were anesthetized 24 h post-Carrageenan administration and bronchoalveolar lavage (BAL) fluid, blood, and lung tissues were harvested.
Bronchoalveolar lavage fluid (BALF) analysis
BAL was collected from anesthetized mice through a 20-gage angiocath as previously described (Gurusamy et al. 2021). Briefly, 4 ml of sterile PBS was instilled into the rat lung and lavaged three times. Cell numbers were standardized/ml BAL recovered. BAL recovery volume range was 3.4–3.7 ml. The cell-free supernatant was stored at -80 to determine the level of cytokines and total protein content. BAL cell counts were determined using a standard hemocytometer. Differential cell counts were subsequently performed on Giemsa-wright stained (Microscopy Hemacolor-Merck; Germany) cytospin preparations. Cell numbers were standardized/ml of BAL collected and results were expressed as number/ml × total volume.
Measurement of wet to dry ratio of the lungs
The lower lobes of the right lung were harvested in all animals to determine the edema index (wet/dry ratio) as previously described (Gurusamy et al. 2021). In brief, The wet weight of the right lower lungs was measured at the time scarification. The dry weight was determined after lungs were incubated for 48 h at 80 °C in an oven. Finally, the W/D ratio was calculated.
Lung pathology
One part of the upper left lung was fixed in 10% formalin. Formalin-fixed paraffin-embedded tissue was sectioned (5 μm thick), hematoxylin- and eosin-stained, and analyzed under a light microscope. Three sections per lung tissue were assessed for each rat, with ten areas per section analyzed. A trained pathologist who was blinded to experimental groups/treatments scored the degree of lung injury using a 5-point scoring system measuring edema, neutrophil infiltration, hemorrhage, and disorganization of lung parenchyma as previously described (Honari et al. 2021). Higher scores indicate more severe lung abnormalities: 0 = normal, 1 = light, 2 = moderate, 3 = severe, and 4 = very severe(Nasseri et al. 2015). Scores for each of the four categories were combined to provide a total lung injury score.
Measurements of inflammatory cytokines in BALF
50 μl of BALF supernatant was used to measure the total concentration of inflammatory mediators. Enzyme immunoassay commercially available kits for rat IL-6 and TNF-α (R & D Systems Inc., Minneapolis, MN, USA) were used to determine BAL fluid concentrations of these meditators according to the manufacturer’s instruction.
Measurements of protein concentration in BALF
BALF supernatant was used to measure protein concentration. The protein of BALF was measured by a Bradford detection kit (Kiazist, Iran).
Serum levels of lipid peroxidation, total antioxidant capacity, and thiol
Lipid peroxidation, total antioxidant capacity (TAC), and the level of thiol were measured according to the company’s instruction [ZANTOX (K.A.A), Birjand, Iran]. Lipid peroxidation was measured according to the thiobarbituric acid reactive substance (TBARs) method. Total antioxidant capacity was measured by a ferric reducing antioxidant power (FRAP) assay. Elman method was used to measure thiol groups.
Statistical analysis
The results were reported in terms of mean ± standard error. Statistical tests used included one-way ANOVA and Tukey test. The Significance limit was defined as (P < 0.05). All statistical analyses were performed using SPSS software version 16.
Results
EA and NAC reduced immune cell influx into the lungs of carrageenan-challenged rats
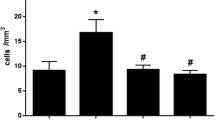
Intratracheal carrageenan injection resulted in a marked increase in lung permeability as evidenced by excessive total inflammatory cell infiltration and neutrophils in the carrageenan group, compared with the control animals(P < 0.001). Treatment with EA extract (100 mg/kg) and NAC (50 mg/kg) however, significantly decreased BALF’ total cell influx (Fig. 1A). Neutrophil counts were significantly reduced in all treated groups compared with carrageenan animals (P < 0.05) (Fig. 1B). The number of total cellular infiltrates, and neutrophils in the BAL fluid was reduced by 58% and 27%, respectively, in rats treated with EA extract (100 mg/kg), 45% and 19% reduction in animals treated with NAC (50 mg/kg) compared with carrageenan group.
The effects of NAC and Elaeagnus angustifolia on carrageenan-induced lung injury. Values are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the carrageenan-induced lung injury group. A Total cell count in BALF. B Neutrophils counts in BALF
EA and NAC attenuated interstitial edema of carrageenan-challenged rats
Administration of Carrageenan into the trachea resulted in a marked increase in lung permeability as evidenced by an increase in lung’s water content. The Carrageenan group indicated a significant increase in wet to dry ratio (W/D) compared to the control group. Meanwhile, EA extract (100 mg/kg) and NAC (50 mg/kg) treatments, profoundly reduced W/D ratio by 67% and 69% respectively (P < 0.05)( Fig. 2A).
The effects of NAC and Elaeagnus angustifolia on carrageenan-induced lung injury. Values are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the carrageenan-induced lung injury group. A lung wet/dry (W/D) weight ratio of rat in each group. B the comparison of lung injury scoring among the groups. C Pathological changes of lung tissues of rat detected by H&E staining
EA and NAC attenuated lung’s histological destruction induced by carrageenan
Intratracheal carrageenan injection-induced ALI characterized by destruction of lung architecture, increased cell infiltration, and hemorrhage compared with the control group (Fig. 2C). Destruction of lung architecture as evidenced by significantly increased lung histology scores (Fig. 2B). All carrageenan-induced pathological changes were attenuated in rats treated with EA extract (100 mg/kg) and NAC (50 mg/kg) by 47% and 57%, respectively (P < 0.05).
EA and NAC ameliorated BAL’s levels of Inflammatory cytokines induced by carrageenan
To determine the impact of EA and NAC treatments on Carrageenan-induced ALI, the levels of TNF-α and IL-6 into the BAL fluid were measured. Post intratracheal carrageenan injection, Inflammatory cell influx into the airways was accompanied by elevated levels of both inflammatory mediators, TNF-α and IL-6, in comparison with the control rats (P < 0.001) (Fig. 3A, B). EA extracts (100 and 400 mg/kg) and NAC (50 and 200 mg/kg) could significantly ameliorate TNF-α levels, while only with EA extract (100 mg/kg) and NAC (50 mg/kg) reduced IL-6 levels to a significant degree compared with carrageenan-treated animals.
The effects of NAC and Elaeagnus angustifolia on carrageenan-induced lung injury. Values are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the carrageenan-induced lung injury group. A Tumor necrosis factor alpha (TNF-α) in BALF. B Interleukin-6 (IL-6) levels in BALF. C Protein concentration in BALF
EA and NAC reduced BALF total protein content induced by carrageenan
Total protein concentration in the carrageenan group significantly increased (P < 0.001) compared to the control group. Treatment with EA extract (100 and 400 mg/kg) and NAC (50 and 200 mg/kg); however, profoundly attenuated the presence of total protein content in BAL compared to the carrageenan only rats. significant (P < 0.001) showed a decrease in the protein concentration compared to the carrageenan group. (Fig. 3C).
EA and NAC reduced carrageenan-induced levels of serum lipid peroxidation, total antioxidant capacity, and thiol
Intratracheal administration of carrageenan significantly elevated the amount of lipid peroxidation and reduced total antioxidant capacity and thiol contents in serum samples. The MDA test showed the rate of lipid peroxidation increased significantly in the carrageenan-treated group compared to the control animals (P < 0.05) (Fig. 4A). EA (400 mg/kg), NAC (200 mg/kg) and EA (400 mg/kg) + NAC (200 mg/kg) administrations, however attenuated the amounts of lipid peroxidation. Furthermore, the FRAP levels (antioxidant capacity) in the carrageenan group were significantly reduced compared to the control rats (Fig. 4B). This situation was reversed via EA (400 mg/kg), NAC (200 mg/kg) and EA (400 mg/kg) + NAC (200 mg/kg) treatments (P < 0.05). Finally, the thiol content of the carrageenan rats was profoundly decreased compared to the control group (P < 0.001). Administration of NAC (200 mg/kg) and EA (400 mg/kg) + NAC (200 mg/kg) however, ameliorated thiol content of the serum (P < 0.05) (Fig. 4C).
The effects of NAC and Elaeagnus angustifolia on carrageenan-induced lung injury. Values are presented as mean ± SEM (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 compared to the control group; #P < 0.05, ##P < 0.01, ###P < 0.001 compared to the carrageenan-induced lung injury group. A lipid peroxidation (MDA) in serum B Total antioxidant capacity (TAC) in serum.C Thiol content in serum
Discussion
Acute lung injury refers to activating the immune responses in the lungs. During lung inflammation, immune cells are activated, resulting in increased inflammatory cytokines such as IL-6 and TNF-α (Pedersen 2017; Ye et al. 2019). Our results in the present study demonstrated that carrageenan-mediated ALI induction can cause neutrophilic infiltration, hemorrhage, and increased lung tissue injury. In addition, carrageenan elevated the concentration of infiltrated total proteins, TNF-α and IL-6 in BALF. The underlying mechanisms for acute lung injury as a devastating disease and its more severe form, ARDS, are complex. Meawhile, it is suggested that oxidative stress and systemic inflammatory responses may play an important role in the development of ALI/ARDS (Tasaka et al. 2008). Oxidative stress is induced by an imbalance of conditions between the production of oxygen free radicals and the body’s antioxidant capacity, which can subsequently activate inflammatory cells leading to serious disorder of ALI (Tasaka et al. 2008; Yang et al. 2019). Inflammation involves multiple cellular and molecular mechanisms in which various mediators participate effectively and appropriately (Medzhitov 2008; Adefegha et al. 2017). Inhibiting both inflammation and oxidative stress therefore considered as effective strategies in ameliorating the ALI/ARDS symptoms (Chen et al. 2017). Carrageenan-induced ALI is broadly used to analyze inflammatory responses (Ekuadzi et al. 2018). In this model, the development of oxidative stress and inflammation are jointly participate via neutrophil infiltration, overproduction of ROS, and generation of inflammatory cytokines. (Yang et al. 2020). The present study confirmed that Elaeagnus angustifolia and N-Acetylcysteine decreased the levels of inflammatory mediators TNF-α and IL-6 in a significant manner. Assessment of the degree of lung damage also demonstrated that EA and NAC ameliorated detrimental effects of carrageenan in reducing lung damage, inflammatory cytokines, and preventing neutrophil infiltration. The pro-inflammatory cytokines TNF-α and IL-6 are mainly secreted by neutrophils, play a major role in initiating and progressing inflammatory responses initially via the overproduction of adhesion molecules (de Campos Facchin et al. 2020). Previous studies have shown that the migration of neutrophils from the blood into the cavity and chest tissues plays an important role in producing pro-inflammatory adhesion molecules (ICAM-1 and VCAM-1) (Gao et al. 2020). The induction of pro-inflammatory cytokines such as TNF-α and IL-6 in ALI model is mainly mediated by the action of carrageenan and other high affinity ligands on toll-like receptor 4 (TLR4). As a pattern recognition receptor (PRR), TLR4 is responsible for the activation of various intracellular pathways, such as the NF-κB and p38 MAPK (Bhattacharyya et al. 2008). Carrageenan have demonstrated to mediated NF-κB activation via subsequent phosphorylation of the inhibitor of NF-κB (IκB) in the cytosol and rapidly degrades IκB through the ubiquitin–proteasome pathway. The p65 phosphorylated subunit of NF-κB in turn causes the overproduction of TNF-α, IL-1β, IL-6, and other pro-inflammatory mediators (Cho et al. 2009; Pereira dos Santos Nascimento et al. 2016). It’s reported that SARS-CoV-2 and other high affinity ligands for TLR4 might induce-NF-kB activation in a similar manner (Kircheis et al. 2020). Consistent with the present report, carrageenan-induced pleurisy represented similar inflammatory responses as increased levels of cytokines, induction of neutrophil migration, and increase in total protein content (Dos Santos et al. 2021; Bezerra Rodrigues Dantas et al. 2020). In line with our study, NAC has been shown to inhibit the lipopolysaccharide-induced TNF-α and IL-6 levels in the inflammatory response in bone marrow mesenchymal stem cells (Wang et al. 2020). N-acetylcysteine can also prevent lung inflammation and neutrophil infiltration after short-term inhalation exposure to concentrated ambient particles (Rhoden et al. 2004). Besides the main anti-inflammatory therapeutic effects of NAC, this compound is well known as a mucolytic drug, anti-pulmonary fibrosis, and a hepatic protective agent (Schwalfenberg, 2021). Recently, NAC has been shown to be effective against neutrophilic airway inflammation in patients with cystic fibrosis, suppresses ubiquitination and degradation of I-κB (NF-κB inhibitor) and thereby blocking NF-κB nuclear translocation and activation (Ryu et al. 2019) (Pajonk et al. 2002; Pei et al. 2018). Surprisingly, studies have demonstrated the anti-COVID-9 effects of NAC (Poe and Corn 2020; Assimakopoulos and Marangos 2020). It’s shown that NAC may interfere with the binding of SARS-COV-2 to angiotensin-converting enzyme-2 (ACE2) receptor (Poe and Corn 2020; Andreou et al. 2020), suggestive as a promising candidate against COVID-19 to prevent the development of ARDS (Assimakopoulos and Marangos 2020, Cadegiani 2020). Phytochemical studies have shown that EA fruit extract contains flavonoids, terpenoids, and other chemicals (Motevalian et al. 2017; Ahmadiani et al. 2000). Flavonoids and terpenoids have been reported for their anti-inflammatory and inhibitory effects on arachidonic acid metabolism and the activities of pro-inflammatory cytokines and adhesion molecules in vitro (del Carmen Recio et al. 1995) (Ali et al. 2011; Serafini et al. 2010). Besides, the anti-inflammatory activity of Elaeagnus angustifolia fruit extract has been proven on rat paw edema (Motevalian et al. 2017). In our study, serum antioxidant evaluations indicate that carrageenan increased MDA levels and decreased thiol groups and antioxidant capacity (FRAP) in acute lung injury while EA extracts and NAC could reversed aforementioned conditions. The carrageenan-induced inflammatory response is closely associated with decreased antioxidant enzyme activity, free radical production, and lipid peroxidation. Lipid peroxidation acts as a marker of cell damage and induces inflammatory processes(Lu et al. 2007). Similar to our data, a study conducted on carrageenan-induced pleurisy in mice, it was demonstrated that carrageenan increased MDA and decreased GSH (Yang et al. 2020). GSH is the body's main antioxidant with diverse cellular functions. GSH is present in high concentrations in BALF fluid and protects the lungs against oxidative damage caused by internal or external stimuli. Reduction in the lung’s environment is associated with an increased risk of lung injury and disease. One strategy to limit oxidative lung damage is to increase the intracellular glutathione content using its precursors, such as NAC, a compound containing thiol and glutathione groups(Galvão et al. 2011). Studies have shown that thiol-containing antioxidants can prevent the release of inflammatory mediators from epithelial cells, thus increasing intracellular GSH and reducing free radicals and NF-κB activation (Caglikulekci et al. 2006). The NAC eradicates free radicals and reacts directly with its free thiol side chain with electrophilic groups such as hydroxyl radical (OH.), Nitrogen dioxide (NO2.), and carbon dioxide ion (CO3.−) with the eventual consequence of detoxification of ROS produced by leukocytes (Akca et al. 2005). The indirect antioxidant function of NAC relies on intracellular GSH replenishment by inserting cysteine into the GSH biosynthesis pathway (Aldini et al. 2018). A recent study conducted on acute kidney injury in septic rats has depicted, that NAC can reduce the level of MDA and increase antioxidant enzymes followed by an increase in antioxidant capacity (Fan et al. 2020). Plant extracts generally exert their protective effect against oxidative stress damage through increased antioxidant, free radical scavenging and anti-lipid peroxidation capacities due in part to their active compounds of flavonoids, tannins, triterpenoids, and alkaloids (Amini et al. 2019). we have illustrated in the present report the therapeutic efficacy of NAC and EA substances against carrageenan-induced ALI phenotype in Wistar rats in reducing inflammation and oxidative stress from various cellular, immunological and biochemical aspects. Its important to highlight the notion that the generation of different species of ROS is strongly involved in ALI progression and deterioration and herbal extracts as well as NAC are capable of diminishing their detrimental effects (Lu et al. 2019). However, our study suffered from some limitations: Our lack of knowledge regarding précised molecular signaling of EA extract and NAC and their effectiveness in carrageenan-induced ALI was the most important one. On that account, we didn’t evaluate a wide spectrum of involved molecular mechanisms and focused on the main preliminary objectives of the project. Furthure evaluations are necessary to clarify the efficacy of these two therapeutic substances in ALI model.
Conclusions
The results of this project clearly demonstrated a significant reduction in infiltration of inflammatory cells within the lung, reduction of pulmonary edema, reversibility of structural changes in lung tissue, effective reduction of inflammatory cytokines and finally a significant reduction of oxidative stress. Accordingly, EA extract and NAC can be considered promising treatment modalities against ALI/ARDS phenotype.
Data availability statements
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Adefegha SA, Rosa Leal DB, Olabiyi AA, Oboh G, Castilhos LG (2017) Hesperidin attenuates inflammation and oxidative damage in pleural exudates and liver of rat model of pleurisy. Redox Rep 22:563–571
Ahmadiani A, Hosseiny J, Semnanian S, Javan M, Saeedi F, Kamalinejad M, Saremi S (2000) Antinociceptive and anti-inflammatory effects of Elaeagnus angustifolia fruit extract. J Ethnopharmacol 72:287–292
Akca T, Canbaz H, Tataroglu C, Caglikulekci M, Tamer L, Colak T, Kanik A, Bilgin O, Aydin S (2005) The effect of N-acetylcysteine on pulmonary lipid peroxidation and tissue damage. J Surg Res 129:38–45
Alam MB, Ju M-K, Kwon Y-G, Lee SH (2019) Protopine attenuates inflammation stimulated by carrageenan and LPS via the MAPK/NF-κB pathway. Food Chem Toxicol 131:110583
Aldini G, Altomare A, Baron G, Vistoli G, Carini M, Borsani L, Sergio F (2018) N-Acetylcysteine as an antioxidant and disulphide breaking agent: the reasons why. Free Radic Res 52:751–762
Ali MK, Ashraf A, Biswas NN, Karmakar UK, Afroz S (2011) Antinociceptive, anti-inflammatory and antidiarrheal activities of ethanolic calyx extract of Hibiscus sabdariffa Linn.(Malvaceae) in mice. Zhong xi yi jie he xue bao= J Chin Integr Med 9:626–631
Amini MH, Ahmady A, Zhakfar AM, Sediqi MN, Babak G (2019) Preliminary phytochemical profile, in vitro antioxidant and sun protective activities of Alhagi pseudalhagi and Elaeagnus angustifolia L. J Pharm Res Int 31(4):1–13
Andreou A, Trantza S, Filippou D, Sipsas N, Tsiodras S (2020) COVID-19: the potential role of copper and N-acetylcysteine (NAC) in a combination of candidate antiviral treatments against SARS-CoV-2. in vivo 34:1567–1588
Assimakopoulos SF, Marangos M (2020) N-acetyl-cysteine may prevent COVID-19-associated cytokine storm and acute respiratory distress syndrome. Med Hypotheses 140:109778
Bezerra Rodrigues Dantas L, Silva ALM, Da Silva Júnior CP, Alcântara IS, Correia De oliveira MR, Oliveira Brito Pereira Bezerra Martins A, Ribeiro-Filho J, Coutinho HDM, Rocha Santos Passos F, Quintans-Junior LJ (2020) Nootkatone inhibits acute and chronic inflammatory responses in mice. Molecules 25:2181
Bhatia M, Moochhala S (2004) Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol 202:145–156
Bhattacharyya S, Dudeja PK, Tobacman JK (2008) Carrageenan-induced NFκB activation depends on distinct pathways mediated by reactive oxygen species and Hsp27 or by Bcl10. Biochimica et Biophysica Acta (BBA)-General Subjects 1780:973–982
Guo Y, Ha B, Zen K, Liu Y (2012) Regulation of the inflammatory response: enhancing neutrophil infiltration under chronic inflammatory conditions. J Immunol 188:844–853
Cadegiani FA (2020) Repurposing existing drugs for COVID-19: an endocrinology perspective. BMC Endocr Disord 20:1–19
Caglikulekci M, Dirlik M, Pata C, Plasse M, Tamer L, Ogetman Z, Ercan B (2006) Effect of N-acetylcysteine on blood and tissue lipid peroxidation in lipopolysaccharide-induced obstructive jaundice. J Invest Surg 19:175–184
Cao X (2020) COVID-19: immunopathology and its implications for therapy. Nat Rev Immunol 20:269–270
Chen X, Zhang X, Zhang J, Gao Y, Yang Z, Li S, Dai H (2017) Attenuation of acute lung injury in a rat model by Semen Cassiae. BMC Complement Altern Med 17:1–7
Cho W, Nam J-W, Kang H-J, Windono T, Seo E-K, Lee K-T (2009) Zedoarondiol isolated from the rhizoma of Curcuma heyneana is involved in the inhibition of iNOS, COX-2 and pro-inflammatory cytokines via the downregulation of NF-κB pathway in LPS-stimulated murine macrophages. Int Immunopharmacol 9:1049–1057
De Campos Facchin BM, da Rosa JS, Luz ABG, Moon YJK, de Lima TC, Casoti R, Biavatti MW, Dalmarco EM, Fröde TS (2020) Systemic administration of Calea pinnatifida inhibits inflammation induced by carrageenan in a murine model of pulmonary neutrophilia. Mediators Inflamm 2020
de Flora S, Balansky R, la Maestra S (2020) Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J 34:13185–13193
Del Carmen RM, Giner RM, Manez S, Talens A, Cubells L, Gueho J, Julien H, Hostettmann K, Rios J (1995) Anti-inflammatory activity of flavonol glycosides from erythrospermum monticolum depending on single or repeated local TPA administration. Planta Med 61:502–504
Dos Santos E, Silva-Filho SE, Radai JAS, Arena AC, Fraga TL, Cardoso CAL, Croda J, Kassuya CAL (2021) Anti-inflammatory properties of ethanolic extract from Vatairea macrocarpa Leaves. J Ethnopharmacol 278:114308
Ekuadzi E, Biney RP, Benneh CK, OseiAmankwaa B, Jato CK (2018) Antiinflammatory properties of betulinic acid and xylopic acid in the carrageenan-induced pleurisy model of lung inflammation in mice. Phytother Res 32:480–487
Fan H, Le J-W, Zhu J-H (2020) Protective effect of N-acetylcysteine pretreatment on acute kidney injury in septic rats. J Surg Res 254:125–134
Galvão AM, Andrade ADD, Maia MBDS, Silva KERD, Bezerra ADA, Melo JFD, Morais NGD, Costa TBD, Castro CMMBD (2011) Antioxidant supplementation for the treatment of acute lung injury: a meta-analysis. Revista Brasileira De Terapia Intensiva 23:41–48
Gao Y, Lv X, Yang H, Peng L, Ci X (2020) Isoliquiritigenin exerts antioxidative and anti-inflammatory effects via activating the KEAP-1/Nrf2 pathway and inhibiting the NF-κB and NLRP3 pathways in carrageenan-induced pleurisy. Food Funct 11:2522–2534
Gurusamy M, Nasseri S, Rampa DR, Feng H, Lee D, Pekcec A, Doods H, Wu D (2021) Inhibition of microsomal prostaglandin E synthase-1 ameliorates acute lung injury in mice. J Transl Med 19:1–11
Hilda JN, Das SD (2016) TLR stimulation of human neutrophils lead to increased release of MCP-1, MIP-1α, IL-1β, IL-8 and TNF during tuberculosis. Hum Immunol 77:63–67
Honari N, Shaban P, Nasseri S, Hosseini M (2021) Ethanolic extract of Achillea wilhelmsii C. Koch improves pulmonary function and inflammation in LPS-induced acute lung injury mice. J Complement Integr Med
Kircheis R, Haasbach E, Lueftenegger D, Heyken WT, Ocker M, Planz O (2020) NF-κB pathway as a potential target for treatment of critical stage COVID-19 patients. Front Immunol 11:3446
Laffey JG, Matthay MA (2017) Fifty years of research in ARDS. Cell-based therapy for acute respiratory distress syndrome. Biology and potential therapeutic value. Am J Respir Crit Care Med 196:266–273
Lee H-R, Shin S-H, Kim JH, Sohn K-Y, Yoon SY, Kim JW (2019) 1-Palmitoyl-2-linoleoyl-3-acetyl-rac-glycerol (PLAG) rapidly resolves LPS-induced acute lung injury through the effective control of neutrophil recruitment. Front Immunol 10:2177
Li X, Geng M, Peng Y, Meng L, Lu S (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal 10:102–108
Lu T-C, Ko Y-Z, Huang H-W, Hung Y-C, Lin Y-C, Peng W-H (2007) Analgesic and anti-inflammatory activities of aqueous extract from Glycine tomentella root in mice. J Ethnopharmacol 113:142–148
Lu X, Ma Y, He J, Li Y, Zhu H, Yu X (2019) N-acetylcysteine for adults with acute respiratory distress syndrome: a meta-analysis of randomized controlled trials. Hong Kong J Emerg Med 26(5):288–298
MEDZHITOV, R. (2008) Origin and physiological roles of inflammation. Nature 454:428–435
Motevalian M, Shiri M, Shiri S, Shiri Z, Shiri H (2017) Anti-inflammatory activity of Elaeagnus angustifolia fruit extract on rat paw edema. J Basic Clin Physiol Pharmacol 28:377–381
Nasseri S, Gurusamy M, Jung B, Lee D, Khang G, Doods H, Wu D (2015) Kinin B1 receptor antagonist BI113823 reduces acute lung injury. Crit Care Med 43:e499–e507
Okmen G, Turkcan O (2014) A study on antimicrobial, antioxidant and antimutagenic activities of Elaeagnus angustifolia L. leaves. Afr J Tradit Complement Altern Med 11:116–120
Ou Z, Zhao J, Zhu L, Huang L, Ma Y, Ma C, Luo C, Zhu Z, Yuan Z, Wu J (2019) Anti-inflammatory effect and potential mechanism of betulinic acid on λ-carrageenan-induced paw edema in mice. Biomed Pharmacother 118:109347
Pajonk F, Riess K, Sommer A, McBride WH (2002) N-acetyl-L-cysteine inhibits 26S proteasome function: implications for effects on NF-κB activation. Free Radical Biol Med 32:536–543
PEDERSEN, B. K. (2017) Anti-inflammatory effects of exercise: role in diabetes and cardiovascular disease. Eur J Clin Invest 47:600–611
Pei Y, Liu H, Yang Y, Yang Y, Jiao Y, Tay FR, Chen J (2018) Biological activities and potential oral applications of N-acetylcysteine: progress and prospects. Oxidative Med Cell Longev
Dos Santos P, Nascimento MV, Arruda-Silva F, Gobbo Luz AB, Baratto B, Venzke D, Mendes BG, Fröde TS, Geraldo Pizzolatti M, Dalmarco EM (2016) Inhibition of the NF-κB and p38 MAPK pathways by scopoletin reduce the inflammation caused by carrageenan in the mouse model of pleurisy. Immunopharmacol Immunotoxicol 38:344–352
Poe FL, Corn J (2020) N-acetylcysteine: a potential therapeutic agent for SARS-CoV-2. Med Hypotheses 143:109862
Qiao J, Chen L, Huang X, Guo F (2019) Effects of nebulized N-acetylcystein on the expression of HMGB1 and RAGE in rats with hyperoxia-induced lung injury. J Cell Physiol 234:10547–10553
Rhoden CR, Lawrence J, Godleski JJ, González-Flecha B (2004) N-acetylcysteine prevents lung inflammation after short-term inhalation exposure to concentrated ambient particles. Toxicol Sci 79:296–303
Ryu C-M, Shin JH, Yu HY, Ju H, Kim S, Lim J, Heo J, Lee S, Shin D-M, Choo M-S (2019) N-acetylcysteine prevents bladder tissue fibrosis in a lipopolysaccharide-induced cystitis rat model. Sci Rep 9:1–11
Schwalfenberg GK (2021) N-Acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J Nutr Metabol
Serafini M, Peluso I, Raguzzini A (2010) Flavonoids as anti-inflammatory agents. Proc Nutr Soc 69:273–278
Shen Y, Miao N-J, Xu J-L, Gan X-X, Xu D, Zhou L, Xue H, Zhang W, Lu L-M (2016) N-acetylcysteine alleviates angiotensin II-mediated renal fibrosis in mouse obstructed kidneys. Acta Pharmacol Sin 37:637–644
Tasaka S, Amaya F, Hashimoto S, Ishizaka A (2008) Roles of oxidants and redox signaling in the pathogenesis of acute respiratory distress syndrome. Antioxid Redox Signal 10:739–754
Tehranizadeh ZA, Baratian A, Hosseinzadeh H (2016) Russian olive (Elaeagnus angustifolia) as a herbal healer. BioImpacts 6:155
Wang X, Jiang M, He X, Zhang B, Peng W, Guo L (2020) N-acetyl cysteine inhibits the lipopolysaccharide-induced inflammatory response in bone marrow mesenchymal stem cells by suppressing the TXNIP/NLRP3/IL-1β signaling pathway. Mol Med Rep 22:3299–3306
Wu Y, Wang Y, Gong S, Tang J, Zhang J, Li F, Yu B, Zhang Y, Kou J (2020) Ruscogenin alleviates LPS-induced pulmonary endothelial cell apoptosis by suppressing TLR4 signaling. Biomed Pharmacother 125:109868
Yang H, Huang J, Gao Y, Wen Z, Peng L, Ci X (2020) Oridonin attenuates carrageenan-induced pleurisy via activation of the KEAP-1/Nrf2 pathway and inhibition of the TXNIP/NLRP3 and NF-κB pathway in mice. Inflammopharmacology 28:513–523
Yang H, Lv H, Li H, Ci X, Peng L (2019) Oridonin protects LPS-induced acute lung injury by modulating Nrf2-mediated oxidative stress and Nrf2-independent NLRP3 and NF-κB pathways. Cell Commun Signal 17:1–15
Ye J, Guan M, Lu Y, Zhang D, Li C, Zhou C (2019) Arbutin attenuates LPS-induced lung injury via Sirt1/Nrf2/NF-κBp65 pathway. Pulm Pharmacol Ther 54:53–59
Ye R, Liu Z (2020) ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp Mol Pathol 113:104350
Acknowledgements
The authors appreciate the Vice Chancellor for Research and Technology of Birjand University of Medical Sciences for funding this study and providing the necessary facilities.
Funding
Birjand University of Medical Sciences (Grant No: 456321).
Author information
Authors and Affiliations
Contributions
MM: investigation, methodology, data curation, formal analysis, software, writing—original draft. SN: conceptualization, investigation, methodology, formal analysis, writing—review and editing. YM: investigation, methodology, data curation, formal analysis, software. SA: formal analysis, histopathology. AZ: conceptualization, methodology, formal analysis, software, writing—review and editing, funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest and are fully responsible for data collection, originality, interpretation, and writing of this manuscript.
Informed consent
Not applicable.
Ethical approval
All procedures involving animals were in accordance with the national guides in care and use of Laboratory Animals in Scientific Affairs provided by the Iranian Ministry of Health and Medical Education (2019). The guideline is following 1964 Helsinki declaration and its later amendments or comparable ethical standards. Moreover, the animal experiments were approved by the Biriand University of Medical Sciences Ethics Committee (permit code: IR.BUMS.REC.1399.474).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mamashli, M., Nasseri, S., Mohammadi, Y. et al. Anti-inflammatory effects of N-Acetylcysteine and Elaeagnus angustifolia extract on acute lung injury induced by λ-carrageenan in rat. Inflammopharmacol 30, 1759–1768 (2022). https://doi.org/10.1007/s10787-022-01003-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-022-01003-0