Abstract
A model-based experimental approach is presented to measure concentration-dependent diffusion coefficients of binary gases from a single experimental run. The diffusion experiments are performed with a Loschmidt cell combined with holographic interferometry that has been improved in Part I of this paper (Wolff et al. in Int. J. Thermophys. 2018, https://doi.org/10.1007/s10765-018-2450-8). Measurements are taken with the system helium–krypton. Besides highly accurate measurements, a highly accurate diffusion model is required to retrieve the weak concentration dependence of the diffusion coefficient. We derive a consistent diffusion model considering real gas effects and the concentration dependence of the diffusion coefficient. The model describes the experimental fringe data with deviations of less than 0.2 interference fringe orders, which corresponds to a relative deviation of 0.17 % indicating high quality of both the experimental data and the employed model. Therefore, the concentration dependence of the helium–krypton diffusion coefficient could be successfully retrieved from a single experiment of mixing pure gases. Thus, the presented approach allows for the efficient characterization of diffusion in gases.




Similar content being viewed by others
References
E.L. Cussler, Diffusion—Mass Transfer in Fluid Systems (Cambridge University Press, Cambridge, 2007)
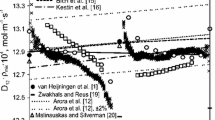
R.J.J. van Heijningen, J.P. Harpe, J.J.M. Beenakker, Physica 38, 1 (1968). https://doi.org/10.1016/0031-8914(68)90059-1
G.R. Staker, P.J. Dunlop, K.R. Harris, T.N. Bell, Chem. Phys. Lett. 32, 561 (1975). https://doi.org/10.1016/0009-2614(75)85240-7
P.J. Carson, P.J. Dunlop, Chem. Phys. Lett. 14, 377 (1972). https://doi.org/10.1016/0009-2614(72)80137-4
G.R. Staker, M.A. Yabsley, J.M. Symons, P.J. Dunlop, J. Chem. Soc. Farad. Trans. 1, 825 (1974). https://doi.org/10.1039/F19747000825
P.J. Carson, P.J. Dunlop, T.N. Bell, J. Chem. Phys. 56, 531 (1972). https://doi.org/10.1063/1.1676900
P.S. Arora, P.J. Carson, P.J. Dunlop, Chem. Phys. Lett. 54, 117 (1978). https://doi.org/10.1016/0009-2614(78)85678-4
T.R. Marrero, E.A. Mason, J. Phys. Chem. Ref. Data 1, 3 (1972). https://doi.org/10.1063/1.3253094
L. Wolff, P. Zangi, T. Brands, M.H. Rausch, H.J. Koß, A.P. Fröba, A. Bardow, Int. J. Thermophys. (2018). https://doi.org/10.1007/s10765-018-2450-8
P.K. Gupta, A.R. Cooper, Physica 54, 39 (1971)
J.C. Clunie, N. Li, M.T. Emerson, J.K. Baird, J. Phys. Chem. 94, 6099 (1990). https://doi.org/10.1021/j100378a085
C. Durou, C. Moutou, J. Mahenc, J. Chim. Phys. 71, 271 (1974). https://doi.org/10.1051/jcp/1974710271
A. Bardow, V. Göke, H.J. Koß, K. Lucas, W. Marquardt, Fluid Phase Equilib. 228, 357 (2005). https://doi.org/10.1016/j.fluid.2004.08.017
E. Kriesten, M.A. Voda, A. Bardow, V. Göke, F. Casanova, B. Blümich, H.J. Koß, W. Marquardt, Fluid Phase Equilib. 277, 96 (2009). https://doi.org/10.1016/j.fluid.2008.10.012
A. Bouchaudy, C. Loussert, J.B. Salmon, AIChE J. (2017). https://doi.org/10.1002/aic.15890
T. Kugler, B. Jäger, E. Bich, M.H. Rausch, A.P. Fröba, Int. J. Thermophys. 34, 47 (2013). https://doi.org/10.1007/s10765-012-1352-4
T. Kugler, M.H. Rausch, A.P. Fröba, Int. J. Thermophys. 36, 3169 (2015). https://doi.org/10.1007/s10765-015-1981-5
M. Kullnick, Interferometrische Untersuchung der Diffusion in binären Gemischen realer Gase mit einer Loschmidt-Diffusionsapparatur. Dissertation, Technical University of Braunschweig (2001)
J. Baranski, Bestimmung binärer Diffusionskoeffizienten von Gasen mit einer Loschmidt-Zelle und holografischer Interferometrie. Dissertation, Universität Rostock, Rostock (2002)
D. Buttig, E. Vogel, E. Bich, E. Hassel, Meas. Sci. Technol. 22, 105409 (2011). https://doi.org/10.1088/0957-0233/22/10/105409
T. Kugler, B. Jäger, E. Bich, M.H. Rausch, A.P. Fröba, Int. J. Thermophys. 36, 3116 (2015). https://doi.org/10.1007/s10765-015-1966-4
J. Crank, The Mathematics of Diffusion (Clarendon Press, Oxford, 1975)
J. Bausa, W. Marquardt, AIChE J. 47, 1318 (2001). https://doi.org/10.1002/aic.690470610
J. Gmehling, B. Kolbe, M. Kleiber, J. Rarey, Chemical Thermodynamics for Process Simulation (Wiley, Hoboken, 2012)
Y. Bard, Nonlinear Parameter Estimation (Academic Press, New York, 1973)
B.N. Srivastava, R. Paul, Physica 28, 646 (1962). https://doi.org/10.1016/0031-8914(62)90120-9
J. Kestin, K. Knierim, E.A. Mason, B. Najafi, S.T. Ro, M. Waldman, J. Phys. Chem. Ref. Data 13, 229 (1984). https://doi.org/10.1063/1.555703
W. Hogervorst, Physica 51, 59 (1971). https://doi.org/10.1016/0031-8914(71)90137-6
G.R. Staker, P.J. Dunlop, Chem. Phys. Lett. 42, 419 (1976). https://doi.org/10.1016/0009-2614(76)80643-4
T. Kugler, Determination of Gaseous Binary Diffusion Coefficients Using a Loschmidt Cell Combined with Holographic Interferometry. Dissertation, Universität Erlangen-Nürnberg (2015)
H.J. Achtermann, J.G. Hong, G. Magnus, R.A. Aziz, M.J. Slaman, J. Chem. Phys. 98, 2308 (1993). https://doi.org/10.1063/1.464212
D.R. MacQuigg, Appl. Opt. 16, 291 (1977). https://doi.org/10.1364/AO.16.000291
D.B. Neumann, H.W. Rose, Appl. Opt. 6, 1097 (1967). https://doi.org/10.1364/AO.6.001097
Acknowledgements
This work was supported by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG) with Grants BA 2884/7-1 and FR 1709/13-1.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Materials:
See Supplementary Material for numerical values of diffusion coefficients from our experiments and from literature. (pdf 152KB)
Rights and permissions
About this article
Cite this article
Wolff, L., Zangi, P., Brands, T. et al. Concentration-Dependent Diffusion Coefficients of Binary Gas Mixtures Using a Loschmidt Cell with Holographic Interferometry. Int J Thermophys 39, 132 (2018). https://doi.org/10.1007/s10765-018-2451-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-018-2451-7