Abstract
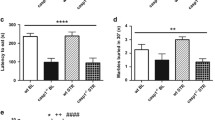
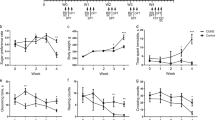
Accumulating evidence has shown that inflammation, the gut microbiota, and neurotransmitters are closely associated with the pathophysiology of depression. However, the links between the gut microbiota and neurotransmitter metabolism remain poorly understood. The present study aimed to investigate the neuroinflammatory reactions in chronic restraint stress (CRS)-induced depression and to delineate the potential links between the gut microbiota and neurotransmitter metabolism. C57BL/6 mice were subjected to chronic restraint stress for 5 weeks, followed by behavioural tests (the sucrose preference test, forced swim test, open field test, and elevated plus maze) and analysis. The results showed that CRS significantly increased interleukin-1 beta (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), and tumour necrosis factor α (TNFα) levels and decreased brain-derived neurotrophic factor (BDNF) expression, accompanied by the activation of IkappaB-alpha-phosphorylation-nuclear factor kappa-B (IκBα-p-NF-κB) signalling in the mouse hippocampus. In addition, the neurotransmitter metabolomics results showed that CRS resulted in decreased levels of plasma 5-hydroxytryptamine (5-HT), dopamine (DA), and noradrenaline (NE) and their corresponding metabolites, and gut microbiota faecal metabolites with the 16S rRNA gene sequencing indicated that CRS caused marked microbiota dysbiosis in mice, with a significant increase in Helicobacter, Lactobacillus, and Oscillibacter and a decrease in Parabacteroides, Ruminococcus, and Prevotella. Notably, CRS-induced depressive behaviours and the disturbance of neurotransmitter metabolism and microbiota dysbiosis can be substantially restored by dexamethasone (DXMS) administration. Furthermore, a Pearson heatmap focusing on correlations between the microbiota, behaviours, and neurotransmitters showed that Helicobacter, Lactobacillus, and Oscillibacter were positively correlated with depressive behaviours but were negatively correlated with neurotransmitter metabolism, and Parabacteroides and Ruminococcus were negatively correlated with depressive behaviours but were positively correlated with neurotransmitter metabolism. Taken together, the results suggest that inflammation is involved in microbiota dysbiosis and the disturbance of neurotransmitter metabolism in CRS-induced depressive changes, and the delineation of the potential links between the microbiota and neurotransmitter metabolism will provide novel strategies for depression treatment.






Similar content being viewed by others
DATA AVAILABILITY
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- CRS:
-
Chronic restraint stress
- PFC:
-
Prefrontal cortex
- DXMS:
-
Dexamethasone
- DA:
-
Dopamine
- NE:
-
Norepinephrine
- 5-HT:
-
Serotonin
- BDNF:
-
Brain-derived neurotrophic factor
- SPT:
-
Sucrose preference test
- OFT:
-
Open field test
- EPM:
-
Elevated plus maze
- FST:
-
Forced swim test
- PrL:
-
Prelimbic cortex
- 5-HIAA:
-
5-Hydroxyindoleacetic acid
- MHPG:
-
3-Methoxy-4-hydroxy-phenylglycol
- 3-MT:
-
3-Methoxytyramine
- HVA:
-
Homovanillic acid
References
Global Burden of Disease Study 2013 Collaborators. 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 386 (9995): 743–800.
Gulbins, A., F. Schumacher, K.A. Becker, B. Wilker, M. Soddemann, F. Boldrin, et al. 2018. Antidepressants act by inducing autophagy controlled by sphingomyelin-ceramide. Molecular Psychiatry 23 (12): 2324–2346.
Kessler, R.C., P. Berglund, O. Demler, R. Jin, D. Koretz, K.R. Merikangas, et al. 2003. The epidemiology of major depressive disorder: Results from the National Comorbidity Survey Replication (NCS-R). JAMA 289 (23): 3095–3105.
Roiser, J.P., R. Elliott, and B.J. Sahakian. 2012. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 37 (1): 117–136.
Kenis, G., and M. Maes. 2002. Effects of antidepressants on the production of cytokines. International Journal of Neuropsychopharmacology 5 (4): 401–412.
Miller, A.H., and C.L. Raison. 2016. The role of inflammation in depression: From evolutionary imperative to modern treatment target. Nature Reviews Immunology 16 (1): 22–34.
Hashioka, S., P.L. McGeer, A. Monji, and S. Kanba. 2009. Anti-inflammatory effects of antidepressants: Possibilities for preventives against Alzheimer’s disease. Central Nervous System Agents in Medicinal Chemistry 9 (1): 12–19.
Goshen, I., T. Kreisel, O. Ben-Menachem-Zidon, T. Licht, J. Weidenfeld, T. Ben-Hur, et al. 2008. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry 13 (7): 717–728.
Jiang, P., Y. Guo, R. Dang, M. Yang, D. Liao, H. Li, et al. 2017. Salvianolic acid B protects against lipopolysaccharide-induced behavioral deficits and neuroinflammatory response: Involvement of autophagy and NLRP3 inflammasome. Journal of Neuroinflammation 14 (1): 239.
Ertenli, I., S. Ozer, S. Kiraz, S.B. Apras, A. Akdogan, O. Karadag, et al. 2012. Infliximab, a TNF-α antagonist treatment in patients with ankylosing spondylitis: The impact on depression, anxiety and quality of life level. Rheumatology International 32 (2): 323–330.
Lopresti, A.L., S.D. Hood, and P.D. Drummond. 2012. Multiple antidepressant potential modes of action of curcumin: A review of its anti-inflammatory, monoaminergic, antioxidant, immune-modulating and neuroprotective effects. Journal of Psychopharmacology. (Oxford) 26 (12): 1512–1524.
Koo, J.W., S.J. Russo, D. Ferguson, E.J. Nestler, and R.S. Duman. 2010. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National academy of Sciences of the United States of America 107 (6): 2669–2674.
O’Sullivan, J.B., K.M. Ryan, N.M. Curtin, A. Harkin, and T.J. Connor. 2009. Noradrenaline reuptake inhibitors limit neuroinflammation in rat cortex following a systemic inflammatory challenge: Implications for depression and neurodegeneration. International Journal of Neuropsychopharmacology 12 (5): 687–699.
Martin, C.R., V. Osadchiy, A. Kalani, and E.A. Mayer. 2018. The brain-gut-microbiome axis. Cellular and Molecular Gastroenterology and Hepatology 6 (2): 133–148.
Mayer, E.A., R. Knight, S.K. Mazmanian, J.F. Cryan, and K. Tillisch. 2014. Gut microbes and the brain: Paradigm shift in neuroscience. Journal of Neuroscience 34 (46): 15490–15496.
Aslam, H., J. Green, F.N. Jacka, F. Collier, M. Berk, J. Pasco, et al. 2018. Fermented foods, the gut and mental health: A mechanistic overview with implications for depression and anxiety. Nutrition Neuroscience 1–13.
Luo, S., X. Kong, J.R. Wu, C.Y. Wang, Y. Tian, G. Zheng, et al. 2018. Neuroinflammation in acute hepatic encephalopathy rats: Imaging and therapeutic effectiveness evaluation using 11C-PK11195 and 18F-DPA-714 micro-positron emission tomography. Metabolic Brain Disease 33 (5): 1733–1742.
Luo, Y., B. Zeng, L. Zeng, X. Du, B. Li, R. Huo, et al. 2018. Gut microbiota regulates mouse behaviors through glucocorticoid receptor pathway genes in the hippocampus. Translational Psychiatry 8 (1): 187.
Jiang, H., Z. Ling, Y. Zhang, H. Mao, Z. Ma, Y. Yin, et al. 2015. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behavior & Immunity 48 (4): 186–194.
Guida, F., F. Turco, M. Iannotta, D. De Gregorio, I. Palumbo, G. Sarnelli, et al. 2018. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain, Behavior, and Immunity 67: 230–245.
Li, N., Q. Wang, Y. Wang, A. Sun, Y. Lin, Y. Jin, et al. 2018. Oral probiotics ameliorate the behavioral deficits induced by chronic mild stress in mice via the gut microbiota-inflammation axis. Frontiers in Behavioral Neuroscience 12: 266.
Dinan, T.G., and J.F. Cryan. 2016. Microbes, immunity and behaviour: Psychoneuroimmunology meets the microbiome. Neuropsychopharmacology Official Publication of the American College of Neuropsychopharmacology 42 (1): 178.
Doherty, F.D., S.M. O’Mahony, V.L. Peterson, O. O’Sullivan, F. Crispie, P.D. Cotter, et al. 2017. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behavior & Immunity 68: S0889159117304804.
Wang, Q., M.A. Timberlake 2nd., K. Prall, and Y. Dwivedi. 2017. The recent progress in animal models of depression. Progress in Neuro-Psychopharmacology and Biological Psychiatry 77: 99–109.
Bska, K.J.J., T. Litwin, I. Joniec, A. Ciesielska, A. Przybyłkowski, A. Członkowski, et al. 2004. Dexamethasone protects against dopaminergic neurons damage in a mouse model of Parkinson’s disease. International Immunopharmacology 4 (10): 1307–1318.
Cassol-Jr, O.J., C.M. Comim, F. Petronilho, L.S. Constantino, E.L. Streck, J. Quevedo, et al. 2010. Low dose dexamethasone reverses depressive-like parameters and memory impairment in rats submitted to sepsis. Neuroscience Letters 473 (2): 126–130.
Khalid, A., B.S. Kim, B.A. Seo, S.T. Lee, K.H. Jung, K. Chu, et al. 2016. Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neuroscience 17: 4.
Wang, L., L. Tang, Y. Feng, S. Zhao, M. Han, C. Zhang, G. Yuan, J. Zhu, S. Cao, Q. Wu, L. Li, and Z. Zhang. 2020. A purified membrane protein from Akkermansia muciniphila or the pasteurised bacterium blunts colitis associated tumourigenesis by modulation of CD8(+) T cells in mice. Gut 69 (11): 1988–1997.
Zhu, Y., E.A. Klomparens, S. Guo, and X. Geng. 2019. Neuroinflammation caused by mental stress: The effect of chronic restraint stress and acute repeated social defeat stress in mice. Neurological Research 41 (8): 762–769.
Guo, Y., J. Xie, X. Li, Y. Yuan, L. Zhang, W. Hu, et al. 2018. Antidepressant effects of rosemary extracts associate with anti-inflammatory effect and rebalance of gut microbiota. Frontiers in Pharmacology 9: 1126.
Zha, L., J. Chen, S. Sun, L. Mao, X. Chu, H. Deng, et al. 2014. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-kB pathway. PLoS ONE 9 (9), e107655.
Zhang, Q., M.J. Lenardo, and D. Baltimore. 2017. 30 years of NF-κB: A blossoming of relevance to human pathobiology. Cell 168 (1–2): 37–57.
Yu, G., and B.M. Sharp. 2012. Nicotine modulates multiple regions in the limbic stress network regulating activation of hypophysiotrophic neurons in hypothalamic paraventricular nucleus. Journal of Neurochemistry 122 (3): 628–640.
Mai, N.T., T.V. Tuan, M. Wolbers, D.M. Hoang, T.V. Nga, T.T. Chau, et al. 2009. Immunological and biochemical correlates of adjunctive dexamethasone in Vietnamese adults with bacterial meningitis. Clinical Infectious Diseases 49 (9): 1387–1392.
Meneses, G., G. Gevorkian, A. Florentino, M.A. Bautista, A. Espinosa, G. Acero, et al. 2017. Intranasal delivery of dexamethasone efficiently controls LPS-induced murine neuroinflammation. Clinical and Experimental Immunology 190 (3): 304–314.
Vizuete, A., F. Hansen, E. Negri, M.C. Leite, D.L. de Oliveira, and C.A. Gonçalves. 2018. Effects of dexamethasone on the Li-pilocarpine model of epilepsy: Protection against hippocampal inflammation and astrogliosis. Journal of Neuroinflammation 15 (1): 68.
Zhao, Y., Q. Wang, M. Jia, S. Fu, J. Pan, C. Chu, et al. 2019. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. Journal of Nutritional Biochemistry 64: 61–71.
Pesarico, A.P., G. Sartori, C.A. Brüning, A.C. Mantovani, T. Duarte, G. Zeni, et al. 2016. A novel isoquinoline compound abolishes chronic unpredictable mild stress-induced depressive-like behavior in mice. Behavioural Brain Research 307: 73–83.
Perez-Caballero, L., S. Torres-Sanchez, C. Romero-López-Alberca, F. González-Saiz, J.A. Mico, and E. Berrocoso. 2019. Monoaminergic system and depression. Cell and Tissue Research 377 (1): 107–113.
Guo, Y., J.P. Xie, K. Deng, X. Li, Y. Yuan, Q. Xuan, et al. 2019. Prophylactic effects of bifidobacterium adolescentis on anxiety and depression-like phenotypes after chronic stress: A role of the gut microbiota-inflammation axis. Frontiers in Behavioral Neuroscience 13: 126.
Kim, S.H., J.W. Lim, H. Kim, 2018. Astaxanthin inhibits mitochondrial dysfunction and interleukin-8 expression in helicobacter pylori-infected gastric epithelial cells. Nutrients 10 (9).
McGee, D.J., X.H. Lu, and E.A. Disbrow. 2018. Stomaching the possibility of a pathogenic role for Helicobacter pylori in Parkinson’s disease. Journal of Parkinson’s Disease 8 (3): 367–374.
Cheng, D., H. Chang, S. Ma, J. Guo, G. She, F. Zhang, et al. 2018. Tiansi liquid modulates gut microbiota composition and tryptophankynurenine metabolism in rats with hydrocortisone-induced depression. Molecules 23 (11).
Miettinen, M., T.E. Pietilä, R.A. Kekkonen, M. Kankainen, S. Latvala, J. Pirhonen, et al. 2012. Nonpathogenic Lactobacillus rhamnosus activates the inflammasome and antiviral responses in human macrophages. Gut Microbes 3 (6): 510–522.
Wong, M.L., A. Inserra, M.D. Lewis, C.A. Mastronardi, L. Leong, J. Choo, et al. 2016. Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Molecular Psychiatry 21 (6): 797–805.
Wang, K., M. Liao, N. Zhou, L. Bao, K. Ma, Z. Zheng, et al. 2019. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Reports 26 (1): 222-235.e5.
Gur, T.L., A.V. Palkar, T. Rajasekera, J. Allen, A. Niraula, J. Godbout, et al. 2019. Prenatal stress disrupts social behavior, cortical neurobiology and commensal microbes in adult male offspring. Behavioural Brain Research 359: 886–894.
Naseribafrouei, A., K. Hestad, E. Avershina, M. Sekelja, A. Linløkken, R. Wilson, et al. 2014. Correlation between the human fecal microbiota and depression. Neurogastroenterology & Motility the Official Journal of the European Gastrointestinal Motility Society 26 (8): 1155–1162.
Wąsik, A., E. Możdżeń, I. Romańska, J. Michaluk, and L. Antkiewicz-Michaluk. 2013. Antidepressant-like activity of the endogenous amine, 1-methyl-1,2,3,4-tetrahydroisoquinoline in the behavioral despair test in the rat, and its neurochemical correlates: A comparison with the classical antidepressant, imipramine. European Journal of Pharmacology 700 (1–3): 110–117.
Bruce-Keller, A., J.M. Salbaum, and H.R. Berthoud. 2017. Harnessing gut microbes for mental health: Getting from here to there. Biological Psychiatry 83 (3): S0006322317319029.
Funding
This work was supported by the Nanjing Science and Technology Bureau (201715024) and Key Project supported by Medical Science and technology development Foundation, Nanjing Department of Health (YKK16072).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethics Approval
Animals were treated in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory, and all procedures were approved by the animal ethical and welfare committee of Nanjing Medical University (NJMU).
Consent for Publication.
Approved by all authors.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, Hl., Li, MM., Zhou, MF. et al. Links Between Gut Dysbiosis and Neurotransmitter Disturbance in Chronic Restraint Stress-Induced Depressive Behaviours: the Role of Inflammation. Inflammation 44, 2448–2462 (2021). https://doi.org/10.1007/s10753-021-01514-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01514-y