Abstract
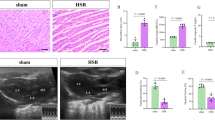
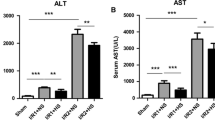
Following hepatic ischemia-reperfusion injury, Kupffer cells could be activated by inflammatory factors released from damaged hepatocytes. Carbon monoxide (CO)-releasing molecule (CORM)-3, a water-soluble transition metal carbonyl, exhibits excellent anti-inflammatory and anti-pyroptosis properties. We investigated whether CORM-3 attenuated hemorrhagic shock and resuscitation (HSR)-induced pyroptosis of Kupffer cells through the soluble guanylate-cyclase (sGC)-cyclic guanosine monophosphate (cGMP) signal pathway. NS2028 (10 mg/kg), a blocker of sGC, was administrated at the onset of hemorrhage, but CORM-3 (4 mg/kg) was infused after resuscitation via femoral vein. Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST) levels, tumor necrosis Factor-α (TNF-α), and interleukin-1β (IL-1β) were measured at 3, 6, 12, and 24 h after HSR, respectively. Six hours post-HSR, liver injury, pyroptosis of Kupffer cells, and expressions in total caspase-1, cleaved caspase-1, gasdermin D (GSDMD) N-terminal fragment, IL-1β, and IL-18 were measured by hematoxylin-eosin (H&E), immunofluorescence and western blot assays, respectively (Fig. 1). The rats exposed to HSR exhibited significant upregulated levels of serum ALT, AST, TNF-α, and IL-1β, elevated liver injury score, increased pyroptosis of Kupffer cells, and accumulated expressions of pyroptosis-associated protein including cleaved caspase-1, GSDMD N-terminal fragment, IL-1β, and IL-18 than sham-treated rats. However, CORM-3 administration markedly reduced liver injury and pyroptosis of Kupffer cells, whereas these protective effects could be partially blocked by NS2028. CORM-3 can mitigate pyroptosis of Kupffer cells in a blood loss and re-infusion model of rats via sGC-cGMP signal pathway.









Similar content being viewed by others
Data Availability
The data used to support the findings of the present study are available from the corresponding author on reasonable request.
References
Montalvo-Jave, E.E., T. Escalante-Tattersfield, J.A. Ortega-Salgado, E. Pina, and D.A. Geller. 2008. Factors in the pathophysiology of the liver ischemia-reperfusion injury. The Journal of Surgical Research 147: 153–159.
Li, C.X., K.T. Ng, Y. Shao, X.B. Liu, C.C. Ling, Y.Y. Ma, et al. 2014. The inhibition of aldose reductase attenuates hepatic ischemia-reperfusion injury through reducing inflammatory response. Annals of Surgery 260: 317–328.
Zhai, Y., R.W. Busuttil, and J.W. Kupiec-Weglinski. 2011. Liver ischemia and reperfusion injury: new insights into mechanisms of innate-adaptive immune-mediated tissue inflammation. American Journal of Transplantation : Official Journal of the American Society of Transplantation and the American Society of Transplant Surgeons 11: 1563–1569.
Cour, M., J. Loufouat, M. Paillard, L. Augeul, J. Goudable, M. Ovize, and L. Argaud. 2011. Inhibition of mitochondrial permeability transition to prevent the post-cardiac arrest syndrome: a pre-clinical study. European Heart Journal 32: 226–235.
Li, J., R.J. Li, G.Y. Lv, and H.Q. Liu. 2015. The mechanisms and strategies to protect from hepatic ischemia-reperfusion injury. European Review for Medical and Pharmacological Sciences 19: 2036–2047.
Cannistra, M., M. Ruggiero, A. Zullo, G. Gallelli, S. Serafini, M. Maria, et al. 2016. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. International Journal of Surgery (London, England) 33 (Suppl 1): S57–S70.
Xi, J., M. Yan, S. Li, H. Song, L. Liu, Z. Shen, and J.Z. Cai. 2019. NOD1 activates autophagy to aggravate hepatic ischemia-reperfusion injury in mice. Journal of Cellular Biochemistry 120: 10605–10612.
Li, S.P., F.F. Wang, W.K. Zhang, M.Z. Bian, S.Y. Zhang, H. Yan, Y. Fang, and H.M. Zhang. 2019. Characteristics of changes in inflammatory cytokines as a function of hepatic ischemia-reperfusion Injury Stage in Mice. Inflammation 42: 2139–2147.
Wu, H.H., C.C. Huang, C.P. Chang, M.T. Lin, K.C. Niu, and Y.F. Tian. 2018. Heat Shock Protein 70 (HSP70) Reduces hepatic inflammatory and oxidative damage in a rat model of liver ischemia/reperfusion injury with hyperbaric oxygen preconditioning. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research 24: 8096–8104.
Ma, G., C. Chen, H. Jiang, Y. Qiu, Y. Li, X. Li, et al. 2017. Ribonuclease attenuates hepatic ischemia reperfusion induced cognitive impairment through the inhibition of inflammatory cytokines in aged mice. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 90: 62–68.
Colino, C.I., J.M. Lanao, and C. Gutierrez-Millan. 2020. Targeting of hepatic macrophages by therapeutic nanoparticles. Frontiers in Immunology 11: 218.
Krenkel, O., and F. Tacke. 2017. Liver macrophages in tissue homeostasis and disease. Nature Reviews Immunology 17: 306–321.
Ryter, S.W., and A.M. Choi. 2016. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Translational Research : the Journal of Laboratory and Clinical Medicine 167: 7–34.
Fujisaki, N., K. Kohama, T. Nishimura, H. Yamashita, M. Ishikawa, A. Kanematsu, T. Yamada, S. Lee, T. Yumoto, K. Tsukahara, J. Kotani, and A. Nakao. 2016. Donor pretreatment with carbon monoxide prevents ischemia/reperfusion injury following heart transplantation in rats. Medical Gas Research 6: 122–129.
Cheng, Y., and J. Rong. 2017. Therapeutic potential of heme oxygenase-1/carbon monoxide system against ischemia-reperfusion Injury. Current Pharmaceutical Design 23: 3884–3898.
Otterbein, L.E., L.L. Mantell, and A.M. Choi. 1999. Carbon monoxide provides protection against hyperoxic lung injury. The American Journal of Physiology 276: L688–L694.
Sato, K., J. Balla, L. Otterbein, R.N. Smith, S. Brouard, Y. Lin, et al. 2001. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. Journal of Immunology (Baltimore, Md. : 1950) 166: 4185–4194.
Zhang, L.M., D.X. Zhang, L. Fu, Y. Li, X.P. Wang, M.M. Qi, C.C. Li, P.P. Song, X.D. Wang, and X.J. Kong. 2019. Carbon monoxide-releasing molecule-3 protects against cortical pyroptosis induced by hemorrhagic shock and resuscitation via mitochondrial regulation. Free Radical Biology & Medicine 141: 299–309.
Suzuki, S., L.H. Toledo-Pereyra, F.J. Rodriguez, and D. Cejalvo. 1993. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation 55: 1265–1272.
Ke, B., X.D. Shen, F. Gao, S. Tsuchihashi, D.G. Farmer, D. Briscoe, R.W. Busuttil, and J.W. Kupiec-Weglinski. 2005. The CD154-CD40 T-cell co-stimulation pathway in liver ischemia and reperfusion inflammatory responses. Transplantation 79: 1078–1083.
Fu, L., D.X. Zhang, L.M. Zhang, Y.C. Song, F.H. Liu, Y. Li, X.P. Wang, W.C. Zheng, X.D. Wang, C.X. Gui, X.J. Kong, and L.Q. Kang. 2020. Exogenous carbon monoxide protects against mitochondrial DNA-induced hippocampal pyroptosis in a model of hemorrhagic shock and resuscitation. International Journal of Molecular Medicine 45: 1176–1186.
Zhang, L.M., and D.X. Zhang. 2019. The dual neuroprotective-neurotoxic effects of sevoflurane after hemorrhagic shock injury. The Journal of Surgical Research 235: 591–599.
Ye, Z., O. Chen, R. Zhang, A. Nakao, D. Fan, T. Zhang, et al. 2015. Methane attenuates hepatic ischemia/reperfusion injury in rats through antiapoptotic, anti-inflammatory, and antioxidative actions. Shock (Augusta, Ga) 44: 181–187.
Sordi, R., F. Chiazza, D. Collotta, G. Migliaretti, R.A. Colas, P. Vulliamy, K. Brohi, J. Dalli, M. Collino, and C. Thiemermann. 2019. Resolvin D1 attenuates the organ injury associated with experimental hemorrhagic shock. Annals of Surgery Publish Ahead of Print.
Schlegel, M., T. Granja, S. Kaiser, A. Korner, J. Henes, K. Konig, et al. 2014. Inhibition of neogenin dampens hepatic ischemia-reperfusion injury. Critical Care Medicine 42: e610–e619.
Zhang, X.J., X. Cheng, Z.Z. Yan, J. Fang, X. Wang, W. Wang, Z.Y. Liu, L.J. Shen, P. Zhang, P.X. Wang, R. Liao, Y.X. Ji, J.Y. Wang, S. Tian, X.Y. Zhu, Y. Zhang, R.F. Tian, L. Wang, X.L. Ma, Z. Huang, Z.G. She, and H. Li. 2018. An ALOX12-12-HETE-GPR31 signaling axis is a key mediator of hepatic ischemia-reperfusion injury. Nature Medicine 24: 73–83.
Xu, Y.J., L. Zheng, Y.W. Hu, and Q. Wang. 2018. Pyroptosis and its relationship to atherosclerosis. Clinica Chimica Acta; International Journal of Clinical Chemistry 476: 28–37.
Mulvihill, E., L. Sborgi, S.A. Mari, M. Pfreundschuh, S. Hiller, and D.J. Müller. 2018. Mechanism of membrane pore formation by human gasdermin-D. The EMBO Journal 37.
Man, S.M., R. Karki, and T.D. Kanneganti. 2017. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological Reviews 277: 61–75.
Chen, X., W.T. He, L. Hu, J. Li, Y. Fang, X. Wang, X. Xu, Z. Wang, K. Huang, and J. Han. 2016. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Research 26: 1007–1020.
Shi, J., Y. Zhao, K. Wang, X. Shi, Y. Wang, H. Huang, Y. Zhuang, T. Cai, F. Wang, and F. Shao. 2015. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526: 660–665.
He, W.T., H. Wan, L. Hu, P. Chen, X. Wang, Z. Huang, Z.H. Yang, C.Q. Zhong, and J. Han. 2015. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Research 25: 1285–1298.
Miao, E.A., J.V. Rajan, and A. Aderem. 2011. Caspase-1-induced pyroptotic cell death. Immunological Reviews 243: 206–214.
Qu, Y., H.L. Zhang, X.P. Zhang, and H.L. Jiang. 2018. Arachidonic acid attenuates brain damage in a rat model of ischemia/reperfusion by inhibiting inflammatory response and oxidative stress. Human & Experimental Toxicology 37: 135–141.
Chen, J., D.M. Zhang, X. Feng, J. Wang, Y.Y. Qin, T. Zhang, Q. Huang, R. Sheng, Z. Chen, M. Li, and Z.H. Qin. 2018. TIGAR inhibits ischemia/reperfusion-induced inflammatory response of astrocytes. Neuropharmacology 131: 377–388.
Zhao, D., S.C. Deng, Y. Ma, Y.H. Hao, and Z.H. Jia. 2018. miR-221 alleviates the inflammatory response and cell apoptosis of neuronal cell through targeting TNFAIP2 in spinal cord ischemia-reperfusion. Neuroreport 29: 655–660.
Ma, Z., Z. Xin, W. Di, X. Yan, X. Li, R.J. Reiter, et al. 2017. Melatonin and mitochondrial function during ischemia/reperfusion injury. Cellular and Molecular Life Sciences: CMLS 74: 3989–3998.
Zhai, Y., H. Petrowsky, J.C. Hong, R.W. Busuttil, and J.W. Kupiec-Weglinski. 2013. Ischaemia-reperfusion injury in liver transplantation--from bench to bedside. Nature Reviews Gastroenterology & Hepatology 10: 79–89.
Li, J., J. Zhao, M. Xu, M. Li, B. Wang, X. Qu, C. Yu, H. Hang, Q. Xia, H. Wu, X. Sun, J. Gu, and X. Kong. 2020. Blocking GSDMD processing in innate immune cells but not in hepatocytes protects hepatic ischemia-reperfusion injury. Cell Death & Disease 11: 244.
Choi, E.Y., S.H. Choe, J.Y. Hyeon, J.I. Choi, I.S. Choi, and S.J. Kim. 2015. Carbon monoxide-releasing molecule-3 suppresses Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1beta in murine macrophages. European Journal of Pharmacology 764: 22–29.
Pizarro, M.D., J.V. Rodriguez, M.E. Mamprin, B.J. Fuller, B.E. Mann, R. Motterlini, and E.E. Guibert. 2009. Protective effects of a carbon monoxide-releasing molecule (CORM-3) during hepatic cold preservation. Cryobiology 58: 248–255.
Motterlini, R., and L.E. Otterbein. 2010. The therapeutic potential of carbon monoxide. Nature Reviews Drug Discovery 9: 728–743.
Ryter, S.W. 2019. Heme oxygenase-1/carbon monoxide as modulators of autophagy and inflammation. Archives of Biochemistry and Biophysics 678: 108186.
Ryter, S.W., K.C. Ma, and A.M.K. Choi. 2018. Carbon monoxide in lung cell physiology and disease. American Journal of Physiology. Cell Physiology 314: C211–c227.
Marazioti, A., M. Bucci, C. Coletta, V. Vellecco, P. Baskaran, C. Szabó, G. Cirino, A.R. Marques, B. Guerreiro, A.M.L. Gonçalves, J.D. Seixas, A. Beuve, C.C. Romão, and A. Papapetropoulos. 2011. Inhibition of nitric oxide-stimulated vasorelaxation by carbon monoxide-releasing molecules. Arteriosclerosis, Thrombosis, and Vascular Biology 31: 2570–2576.
Feng, S., D. Fox, and S.M. Man. 2018. Mechanisms of gasdermin family members in inflammasome signaling and cell death. Journal of Molecular Biology 430: 3068–3080.
Acknowledgements
The authors express their gratitude to Yu-Lin Chang from Cangzhou Central Hospital who helped us with technical support.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81701296 by Dr. Li-Min Zhang).
Author information
Authors and Affiliations
Contributions
Design of the study: Li-Min Zhang
Editing the manuscript: Xu-Peng Wang, Wei-Chao Zheng, and Li-Min Zhang
Statistical analysis: Xu-Peng Wang, Wei-Chao Zheng, and Li-Min Zhang
Experiment and data collection: Xu-Peng Wang, Wei-Chao Zheng, Yue Xin, Yang Bai, Yan Li, Jing-Zhou Wang, and Yu-Lin Chang
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
All animal experiments were carried out in accordance with the National Institute of Health Guideline for the Care and Use of Laboratory Animals and approved by the Animal Review Board of Cangzhou Central Hospital.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, XP., Zheng, WC., Bai, Y. et al. Carbon Monoxide–Releasing Molecule-3 Alleviates Kupffer Cell Pyroptosis Induced by Hemorrhagic Shock and Resuscitation via sGC-cGMP Signal Pathway. Inflammation 44, 1330–1344 (2021). https://doi.org/10.1007/s10753-021-01419-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01419-w