Abstract
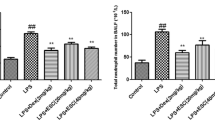
To determine whether low molecular weight heparin (LMWH) is able to reduce pulmonary inflammation and improve the survival in rats with endotoxin-induced acute lung injury (ALI). Rat ALI model was reproduced by injection of lipopolysaccharide (LPS) into tail vein. Rats were divided randomly into three groups: control group, ALI group, LMWH-treated group. Blood was collected and lung tissue was harvested at the designated time points for analysis. The lung specimens were harvested for morphological studies, streptavidin-peroxidase immunohistochemistry examination. Lung tissue edema was evaluated by tissue water content. The levels of lung tissue myeloperoxidase (MPO) were determined. Meanwhile, the nuclear factor-kappa B (NF-κB) activation, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) levels and high mobility group box 1 (HMGB1) and intercellular adhesion molecule-1 (ICAM-1) protein levels in the lung were studied. In survival studies, a separate group of rats were treated with LMWH or sterile saline after LPS administration. Then, the mortality was recorded. Treatment with LMWH after ALI was associated with a reduction in the severity of LPS-induced lung injury. Treatment with LMWH significantly decreased the expression of TNF-α, IL-1β, HMGB1 and ICAM-1 in the lung of ALI rats. Similarly, treatment with LMWH dramatically diminished LPS-induced neutrophil sequestration and markedly reduced the enhanced lung permeability. In the present study, LMWH administration inhibited the nuclear translocation of NF-κB in the lung. Survival was significantly higher among the LMWH-treated group compared with the ALI group. These data suggest that LMWH attenuates inflammation and prevents lethality in endotoxemic rats.








Similar content being viewed by others
Abbreviations
- ALI:
-
acute lung injury
- HMGB1:
-
high mobility group box 1
- ICAM-1:
-
intercellular adhesion molecule-1
- MPO:
-
myeloperoxidase
- NF-κB:
-
nuclear factor-kappa B
- TNF-α:
-
tumor necrosis factor-α
- IL-1β:
-
interleukin-1β
- ELISA:
-
enzyme-linked immunosorbent assay
References
Dreyfuss, D., and G. Saumon. 1998. Ventilator-induced lung injury: lessons from experimental studies. American Journal of Respiratory and Critical Care Medicine 157: 294–323.
Ware, L.B., and M.A. Matthay. 2000. The acute respiratory distress syndrome. New England Journal of Medicine 342: 1334–1349.
Bhatia, M., and S. Moochhala. 2004. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. Journal of Pathology 202: 145–156.
Ghosh, S., R.D. Latimer, B.M. Gray, et al. 1993. Endotoxin-induced organ injury. Critical Care Medicine 21: S19–S24.
Xu, H., X. Ye, H. Steinberg, et al. 2010. Selective blockade of endothelial NF-kappaB pathway differentially affects systemic inflammation and multiple organ dysfunction and injury in septic mice. Journal of Pathology 220: 490–498.
Bustin, M. 1999. Regulation of DNA-dependent activities by the functional motifs of the high-mobility-group chromosomal proteins. Molecular and Cellular Biology 19: 5237–5246.
Wang, H., O. Bloom, M. Zhang, et al. 1999. HMG-1 as a late mediator of endotoxin lethality in mice. Science 285: 248–251.
Reiss, L.K., U. Uhlig, and S. Uhlig. 2012. Models and mechanisms of acute lung injury caused by direct insults. European Journal of Cell Biology 91: 590–601.
Kim, J.Y., J.S. Park, D. Strassheim, et al. 2005. HMGB1 contributes to the development of acute lung injury after hemorrhage. American Journal of Physiology—Lung Cellular and Molecular Physiology 288: L958–L965.
Scaffidi, P., T. Misteli, and M.E. Bianchi. 2002. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 418: 191–195.
Andersson, U., H. Wang, K. Palmblad, et al. 2000. High mobility group 1 protein (HMG-1) stimulates proinflammatory cytokine synthesis in human monocytes. Journal of Experimental Medicine 192: 565–570.
Barsness, K.A., J. Arcaroli, A.H. Harken, et al. 2004. Hemorrhage-induced acute lung injury is TLR-4 dependent. American Journal of Physiology—Regulatory, Integrative and Comparative Physiology 287: R592–R599.
Xie, K., Y. Yu, Y. Huang, et al. 2012. Molecular hydrogen ameliorates lipopolysaccharide-induced acute lung injury in mice through reducing inflammation and apoptosis. Shock 37: 548–555.
Gong, Q., J.F. Xu, H. Yin, et al. 2009. Protective effect of antagonist of high-mobility group box 1 on lipopolysaccharide-induced acute lung injury in mice. Scandinavian Journal of Immunology 69: 29–35.
Wang, H., J.M. Vishnubhakat, O. Bloom, et al. 1999. Proinflammatory cytokines (tumor necrosis factor and interleukin 1) stimulate release of high mobility group protein-1 by pituicytes. Surgery 126: 389–392.
Fiuza, C., M. Bustin, S. Talwar, et al. 2003. Inflammation-promoting activity of HMGB1 on human micro vascular endothelial cells. Blood 101: 2652–2660.
Park, J.S., F. Gamboni-Robertson, Q. He, et al. 2006. High Mobility Group Box 1 protein (HMGB1) interacts with multiple toll like receptors. American Journal of Physiology—Cellular Physiology 290: 917–924.
Kokkola, R., A. Andersson, G. Mullins, et al. 2005. RAGE is the major receptor for the proinflammatory activity of HMGB1 in rodent macrophages. Scandinavian Journal of Immunology 61: 1–9.
Iba, T., A. Kidokoro, and Y. Yagi. 1998. The role of the endothelium in changes in procoagulant activity. Journal of the American College of Surgeons 87: 321–329.
Macfarlane, S.R., M.J. Seatter, T. Kanke, et al. 2001. Proteinase-activated receptors. Pharmacological Reviews 53: 245–282.
Freeman, B.D., B.A. Zehnbauer, and T.G. Buchman. 2003. A meta-analysis of controlled trials of anticoagulant therapies in patients with sepsis. Shock 20: 5–9.
Matthay, M.A. 2001. Severe sepsis: a new treatment with both anticoagulant and anti-inflammatory properties. New England Journal of Medicine 344: 759–762.
Al-Ansari, E., H.K. Du, L. Yu, et al. 2007. Low molecular-weight heparin inhibits hypoxic pulmonary hypertension and vascular remodeling in guinea pigs. Chest 132: 1898–1905.
Li, L.F., C.C. Huang, H.C. Lin, et al. 2009. Unfractionated heparin and enoxaparin reduce high stretch ventilation-augmented lung injury-a prospective, controlled animal experiment. Critical Care 13: R108.
Darien, B.J., J. Fareed, K.S. Centgraf, et al. 1998. Low molecular weight heparin prevents the pulmonary hemodynamic and pathomorphologic effects of endotoxin in a porcine acute lung injury model. Shock 9: 274–281.
Chen, C.M., H.C. Chou, L.F. Wang, et al. 2008. Captopril decreases plasminogen activator inhibitor-1 in rats with ventilatorinduced lung injury. Critical Care Medicine 36: 1880–1885.
Takahashi, H., S. Ebihara, T. Okazaki, et al. 2005. A comparison of the effects of unfractionated heparin, dalteparin and danaparoid on vascular endothelial growth factor-induced tumour angiogenesis and heparinase activity. British Journal of Pharmacology 146: 333–343.
Murakami, K., R. McGuire, R.A. Cox, et al. 2002. Heparin nebulization attenuates acute lung injury in sepsis following smoke inhalation in sheep. Shock 18: 236–241.
Ogawa, Y., K. Yamakawa, H. Ogura, et al. 2012. Recombinant human soluble thrombomodulin improves mortality and respiratory dysfunction in patients with severe sepsis. Journal of Trauma and Acute Care Surgery 72: 1150–1157.
McMaken, S., M.C. Exline, P. Mehta, et al. 2011. Thrombospondin-1 contributes to mortality in murine sepsis through effects on innate immunity. PLoS One 6: e19654.
Levi, Marcel, and Tom van der Poll. 2010. Inflammation and coagulation. Critical Care Medicine 38: S26–S34.
Krzyzaniak, M., G. Cheadle, C. Peterson, et al. 2011. Burn-induced acute lung injury requires a functional toll-like receptor 4. Shock 36: 24–29.
Wang, M., T. Liu, D. Wang, et al. 2011. Therapeutic effects of pyrrolidine dithiocarbamate on acute lung injury in rabbits. Journal of Translational Medicine 9: 61.
Wolfson, R.K., E.T. Chiang, and J.G. Garcia. 2011. HMGB1 induces human lung endothelial cell cytoskeletal rearrangement and barrier disruption. Microvascular Research 81: 189–197.
Ueno, H., T. Matsuda, S. Hashimoto, et al. 2004. Contributions of high mobility group box protein in experimental and clinical acute lung injury. American Journal of Respiratory and Critical Care Medicine 170: 1310–1316.
Angus, D.C., L. Yang, L. Kong, et al. 2007. Circulating high-mobility group box 1 (HMGB1) concentrations are elevated in both uncomplicated pneumonia and pneumonia with severe sepsis. Critical Care Medicine 35: 1061–1067.
Courtine, E., F. Pène, N. Cagnard, et al. 2011. Critical role of cRel subunit of NF-κB in sepsis survival. Infection and Immunity 79: 1848–1854.
Zingarelli, B. 2005. Nuclear factor-kappaB. Critical Care Medicine 33: S414–S416.
Kim, B.H., E. Roh, H.Y. Lee, et al. 2008. Benzoxathiole derivative blocks lipopolysaccharide-induced nuclear factor-kappaB activation and nuclear factor-kappaB-regulated gene transcription through inactivating inhibitory kappaB kinase beta. Molecular Pharmacology 73: 1309–1318.
Ha, T., Y. Xia, X. Liu, et al. 2011. Glucan phosphate attenuates myocardial HMGB1 translocation in severe sepsis through inhibiting NF-κB activation. American Journal of Physiology—Heart and Circulatory Physiology 301: H848–H855.
Esmon, C.T. 2001. Role of coagulation inhibitors in inflammation. Thrombosis and Haemostasis 86: 51–56.
Riewald, M., V.V. Kravchenko, R.J. Petrovan, et al. 2001. Gene induction by coagulation factor Xa is mediated by activation of protease-activated receptor 1. Blood 97: 3109–3116.
Skoutakis, V.A. 1997. Danaparoid in the prevention of thromboembolic complications. Annals of Pharmacotherapy 31: 876–887.
Kwak, H.J., J.S. Song, J.Y. Heo, et al. 2005. Roflumilast inhibits lipopolysaccharide-induced inflammatory mediators via suppression of nuclear factor-kappaB, p38 mitogen-activated protein kinase, and c-Jun NH2-terminal kinase activation. Journal of Pharmacology and Experimental Therapeutics 315: 1188–1195.
Schottelius, A.J., M.W. Mayo, R.B. Sartor, et al. 1999. Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. Journal of Biological Chemistry 274: 31868–31874.
Cavaillon, J.M., C. Marie, M. Caroff, et al. 1996. CD14/LPS receptor exhibits lectin-like properties. Journal of Endotoxin Research 3: 471–480.
Anastase-Ravion, S., C. Blondin, B. Cholley, et al. 2003. Heparin inhibits lipopolysaccharide (LPS) binding to leukocytes and LPS-induced cytokine production. Journal of Biomedical Materials Research. Part A 66: 376–384.
Acknowledgment
The authors thank Dr. Nan Liu (Center of Laboratory Technology and Experimental Medicine; China Medical University) for expert technical support. This work was supported by the National Natural Science Foundation of China (No. 30901438).
Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luan, ZG., Naranpurev, M. & Ma, XC. Treatment of Low Molecular Weight Heparin Inhibits Systemic Inflammation and Prevents Endotoxin-Induced Acute Lung Injury in Rats. Inflammation 37, 924–932 (2014). https://doi.org/10.1007/s10753-014-9812-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-014-9812-6