Abstract
Non-native species’ introductions have increased in the last decades primarily due to anthropogenic causes such as climate change and globalization of trade. Moina macrocopa, a stress-tolerant cladoceran widely used in bioassays and aquaculture, is spreading in temporary and semi-temporary natural ponds outside its natural range. Here, we characterize the variations in the climatic niche of M. macrocopa during its invasions outside the native Palearctic range following introduction into the American continent. Specifically, we examined to what extent the climatic responses of this species have diverged from those characteristics for its native range. We also made predictions for its potential distribution under current and future scenarios. We found that the environmental space occupied by this species in its native and introduced distribution areas shares more characteristics than randomly expected. However, the introduced niche has a high degree of unfilling when displacing its original space towards the extension to drier and hotter conditions. Accordingly, M. macrocopa can invade new areas where it has not yet been recorded in response to warming temperatures and decreasing winter precipitation. In particular, temporary ponds are more vulnerable environments where climatic and environmental stresses may also lower biotic resistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Anthropocene has been a challenge for biodiversity management and conservation of freshwater resources (Reid et al., 2018; Dudgeon, 2019). This “age of mankind” is characterized by widespread environmental disturbances undertaken by several human activities, e.g., climate change, degradation of natural habitats, and biological invasions. The above threats can also enhance biological invasions, which may synergistically threaten biodiversity from species to ecosystems level, thus requiring substantial conservation and management efforts. Therefore, because species distributions are expected to shift with future climates and global trades (Parmesan, 2006; Olden et al., 2021; Wang et al., 2021), more than generating discussions on new paradigms of biogeography concepts and novel ecological hypotheses (Capinha et al., 2015; Hill & Hadly, 2018; Pyšek et al., 2020), we need to forecast the distribution and environmental relatedness of invasive species to then implement adequate monitoring policies.
Assessing species niches and their dynamics can help elucidate alien invasive species distributions and species adaptations to different environmental conditions (Wiens et al., 2009; Tingley et al., 2014). As species distribution models use the ecological characteristics of its known occurrences to estimate suitable areas for the species in its potential distribution area (Peterson & Vieglais, 2001; Cordier et al., 2020), we can theoretically investigate its invasion success and spreading into new areas based on species data and spatial constraints. The assessment of niche conservatism—whether a species may overcome historical constraints and invade previously inaccessible areas (Peterson et al., 1999; Peterson, 2003; Wiens et al., 2010) or niche shifts of invasive populations—whether its success depends on the ability of individuals to undergo new local adaptations not shown in its ancestral niche (Müller-Schärer et al., 2004) is a central question in biological invasions. Explaining the underlying reasons for these individualistic responses requires comparing multiple clades and environmental change types. Accordingly, niche tests complement fundamental assumptions for applying SDMs, assuming that the species occupy similar environmental conditions in new geographical ranges or periods (Pearman et al., 2008).
Currently, some taxonomic groups are adequately investigated in terms of invasion mechanisms involving niche evolution (e.g., plants Broennimann et al., 2007), as for freshwater fishes (Lauzeral et al., 2011), dinoflagellates (Macêdo et al., 2021), and aquatic invertebrates (Torres et al., 2018). However, few studies have combined niche dynamic analysis to changes in species redistribution, making it hard to obtain a general pattern of climate-induced shifts across broad taxonomic spectra (Taheri et al., 2021) making hard to obtain a general pattern of niche dynamics across broad taxonomic spectra. Thus, understanding niche dynamics and mapping the potential distribution of a new invader may provide valuable tools for management actions, particularly if the potential invader (i) has life histories that facilitate colonization (e.g., asexual reproduction; resting stages) (Ruiz et al., 2000), and (ii) if the risk of adverse environmental impacts is high based on taxonomically related species information, especially in the initial stages of invasion (Sousa et al., 2017; Dexter & Bollens, 2019).
Although there is strong literature bias for the environmental effects on invasive microfauna, invasive zooplankton taxa have shown potentialities for exerting moderate to high adverse effects on biodiversity. Specifically, cladocerans are a group of invertebrate animals potentially threatening aquatic biodiversity when acting as invasive species. For example, the invasive Daphnia lumholtzi Sars, 1885 which has a negative impact on other native zooplankton populations (Dzialowski et al., 2000; Soeken-Gittinger et al., 2009). Also, the two invasive predatory cladocerans Bythotrephes longimanus Leydig, 1860 and Cercopagis pengoi Ostroumov, 1891 recognized as major drivers of biodiversity and economic losses (Jacobs & MacIsaac, 2007; Walsh et al., 2016). Among cladocerans, Moina has been intensively studied (Neretina et al., 2020) regarding taxonomy (Alonso et al., 2019), cryptic diversity (Petrusek et al., 2004; Bekker et al., 2016; Montoliu-Elena et al., 2019), and biogeography (Elmoor-Loureiro et al., 2010; Farias et al., 2017). Moinids have also shown morphological similarities with daphniids (Goulden, 1968). However, moinids’ invasion processes are understudied compared to other cladoceran species such as daphniids, despite their relative importance in the Neotropical and Palearctic regions (Forró et al., 2008).
Moina macrocopa Straus, 1820 has been reported as a potential invader of inland waters (Paggi, 1997; Okolodkov et al., 2007). Reported to be native in water bodies of Europe, Africa, the Middle East, and Asia, mainly in shallow temporary lakes, M. macrocopa is claimed to have been introduced in the American continent only more recently due to anthropogenic vectors (Paggi, 1997). However, its invasion pathway and vectors remain largely unknown. In this respect, we set up the framework for evaluating the potentialities of M. macrocopa (Cladocera) as an emerging invasive species and the eventual role of niche evolution to explain its expansion success, while invading new ranges following the transoceanic introductions from the Palearctic region. We tested whether niche conservatism or shift has driven the geographical expansion of M. macrocopa using multivariate analyses, mapping areas suitable for M. macrocopa under current and future climate scenarios (2041–2060) at a global scale. Specifically, as a stress-adapted cladoceran and considering its initial widespread in tropical ponds (an essential detail of its known invasion history), we hypothesized that M. macrocopa would expand its distribution as climate change progresses. We also evaluated environmental predictors of its expected range expansion in the face of the predicted warmer and drier future climate. In doing so, we aim to advance discussion on biogeography and invasion biology of inland water zooplankton and contribute to making policies managers better informed.
Material and methods
Species’ distribution data and curation
We gathered occurrence records from the Global Biodiversity Information Facility (GBIF) (https://doi.org/10.15468/dl.4u4kmf) and a literature review in SCOPUS using the following keywords: “Moina macrocopa” OR “Moina macrocopa.” We performed a broad search based on title, abstract, and keywords, with no additional filter on language or document type. Here, we considered as M. macrocopa s.l., excluding the American clade Moina macrocopa americana Goulden, 1968 as recent research has indicated that these clades are different species (Montoliu-Elena et al., 2019).
We compiled a dataset for further curation using R studio. First, we converted the original data frame into a spatial polygon object, which was then restricted by removing all points outside the extent of the inland buffer shapefile since SDMs must ideally restrict model calibration to accessible areas (Peterson & Soberón, 2012). Furthermore, the occurrence data were checked for missing (NA) values in Longitude and Latitude and for the existence of duplicates for each species subset. We then filtered (spatially thinned) the records using the spThin package (Aiello-Lammens et al., 2015), removing all records with a distance of 5 km between occurrences. The final occurrence dataset (“Mmacro.csv”; Table S1), 95 thinned records (62 invasive and 33 native), and plotted occurrences (“map occur. tiff”; Fig. S1A) are present in Supplementary Material. For the plotted occurrences, we used ArcMap v10.8, using a polygon layer obtained from FEOW.org (Fresh Water Ecoregions of the World; Abell et al., 2008).
Niche test analysis
To predict the environmental space of M. macrocopa, we used all the 19 climatic variables taken from the WorldClim Project (Fick & Hijmans, 2017; http://www.wordclim.org) at a spatial resolution of 10 min (of a longitude/latitude degree). With those variables, we used Broennimann et al. (2012) approach to measure niche conservatism between the native (Palearctic) and the invasive ranges in America. This approach calculates an observed measure of niche overlap and compares it to randomized niche overlap measures. This method calculates the available environmental space, defined by the first two axes from the PCA-env, for each study area (see background areas in Fig. S1B–C) (Broennimann et al., 2012). Later, it measures the niche overlap between native and exotic ranges using Schoener's D metric (Schoener, 1970). This metric varies from 0 to 1, representing totally different or completely overlapping niches, respectively (Broennimann et al., 2012). Then, for the niche overlap, we calculate the D metric and its significance, using a similarity test (based on the 95% confidence interval) which compares the niches in their native and introduced regions (Warren et al. 2008; Broennimann et al., 2012). We repeated each randomization process 100 times, producing a null distribution of overlap values to which the observed score was compared. An observed overlap score that is significantly smaller than one obtained with the null distribution of overlap scores suggests that the species is occupying different environmental spaces in the considered ranges. By doing this, we can investigate the invasion pattern through the niche dynamics and interpret the current knowledge of its genetic comparisons between these areas (Montoliu-Elena et al., 2019).
Modeling fundamental niche
Predictor variables
We used the 19 WorldClim variables at a spatial resolution of 10 min (of a longitude/latitude degree). All environmental layers were clipped to the extent of the study area, resulting in a mask of the world without polar regions. Before processing the models, we carried a multicollinearity test by using the variance inflation factor (VIF) (Marquardt, 1970), a widely used approach to avoid instability in parameter estimation and bias in inference statistics (Dormann et al. 2012). The selected variables (bio02/Mean diurnal range (monthly mean, \(T_{\max }^{^\circ } - T_{\min }^{^\circ }\)), bio08/Mean temperature of wettest quarter, bio09/Mean temperature of driest quarter, bio13/Precipitation of wettest month, bio14/Precipitation of driest month, bio15/Precipitation seasonality (coefficient of variation), bio18/Precipitation of the warmest quarter, bio19/Precipitation of the coldest quarter) that were not highly correlated (Pearson’s R <|0.80|) were considered biologically relevant, or had already been used in other studies and their efficiency demonstrated (Jiménez-Valverde et al., 2011; Palaoro et al., 2013; Sousa et al. 2017).
For future estimations (2041–2060), we used the MIROC6 model from CMIP6 (Coupled Model Intercomparison Project Phase 6), characterized by CO2 and aerosol emission rates (https://www.worldclim.org/data/cmip6/cmip6climate.html#). We generated two future bioclimatic models for two different scenarios of CO2 emission, the optimistic SSP126 and SSP585 (worst case) shared Socio-economic Pathways. We further generated a consensus map from these scenarios. In other words, WorldClim 2.1 provides Global Climate Models (GCM) of the CMIP6 (Tebaldi et al., 2021) and for the four high-priority scenarios, which cover the range of possible pathways depending on socio-economic choices. Specifically, we chose SSP1-2.6—which assumes a “2°C scenario of the sustainability”; and the SSP5-8.5—which refers to a “high reference scenario” in a high fossil-fuel development world throughout the twenty-first century, marking the upper edge of the SSP scenarios (Meinshausen et al., 2020).
Species distribution modeling
We fitted the modeling techniques with species presence data as the response variable and environmental variables as predictors (i.e., explanatory variables). We used algorithms implemented in the SDM R package version 1.0‐67 (Naimi & Araújo, 2016): random forests (RF; Breiman, 2001) and maximum entropy (Maxent; Phillips et al., 2006). Maxent is a correlative model based on the maximum entropy principle for estimating probability distributions that require presence and background data obtained from the whole accessible area. On the other hand area, while RF is a high-performing machine learning technique consisting of multiple decision trees (Breiman, 2001; Olden et al., 2008).
For calibration, 70% of the records (training set) were randomly selected for calibration and the remaining 30% for model evaluation. For each algorithm, ten replicates were employed, using the bootstrapping method. Given that the species occurrence data frame included only presence data, an argument for background data of ten thousand (10,000) points per species using the method “gRandom” was employed (in the script; method = “gRandom,” n = 10,000), with the removal of matching points, to generate pseudo-absence data (Barbet-Massin et al., 2012). Prediction maps were generated from all records (Fig. S1) without distinction between native and invaded areas (Broennimann & Guisan, 2008; Sales et al., 2021). Furthermore, the differentiation between native and non-native ranges is not precise since sampling efforts are lacking globally. The usual method to overcome these issues includes both the native and invaded ranges because such models have better performance than models using only the native range (Broennimann & Guisan, 2008). We evaluated the models using multiple approaches: (1) area under the curve (AUC), in which AUC > 0.9 the predicted model is very good (Swets 1988), (2) Pearson’s correlation coefficient (COR), and (3) explained deviance (deviation). The mean performance is presented in Supplementary Table 1.
Models were summarized in Ensemble maps, using weighted averaging over all predictions from the fitted models (method = “weighted”). In other words, the ensemble combines the prediction of different algorithms and replications to develop a single output. Finally, we visually assessed projected ensemble-based distribution maps (Araújo & New, 2007).
By applying a threshold (i.e., the mean model TSS criteria of model evaluation, “max (se + sp),” the respective maps of binomial probability of occurrence (0 or 1) were obtained. By using the “predict” function (Naimi & Araújo, 2016), the fitted models were used to generate future predictions with the future data layers (per time frame, per SSP scenario). Further, future and present distributions were compared in terms of overall mean probabilities and changes in suitability by mapping areas of decrease, stability, and increase. We performed our analysis in R 4.0.1 (R Core Team, 2021), the extensible R platform for species distribution modeling (Naimi & Araújo, 2016).
Results
According to the multivariate niche analyses, the two niches are more similar than expected randomly (similarity test = 0.02); therefore, we did not reject the niche conservatism hypothesis, although both niches were not identical, showing low observed Schoener’s D = 0.05. We detected high portions of niche unfilling (74%). In other words, more than 70% of the original niche is not filled in the invaded range. The niche expansion was 2.7%, following also high values of stability 97% (Table S2). The centroid of the introduced niche (green) was slightly shifted towards higher precipitation and lower temperatures than that of the native area (orange) (Fig. 1B).
A The resulting PCA with variables available for Moina macrocopa used for the PCA-env approach of Broennimann et al. (2012). B Comparison between the native and exotic ranges of M. macrocopa. Niche occupied by M. macrocopa in its native range (orange), in its invasive range in America (green) and composed niche overlap of both ranges (purple). The continuous line represents the 100% of available environmental background and the dashed line represents the 50% most common conditions. See Figure S2 for the contribution of each variable to the PCA axes
The first two principal components of the nineteen bioclimatic variables for the native and introduced areas explained about 73% of the total variation (Fig. 1A). The first component (53%) grouped the three precipitation-related variables, whereas the second (approx. 20%) grouped the three temperature-related variables (see Fig. S2).
Although the average values of AUC for RF and MaxEnt showed good performance (> 0.8), the ensemble model showed maximizing predictive performance (> 0.9), showing better fit from the weighted overlap of these algorithms (Table S3). Furthermore, the potential invasive risk area predicted by ensembles can cover most of the current distribution records of M. macrocopa used in this study.
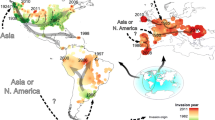
Our distribution modeling revealed that current suitability was considered low in most parts of the Palearctic except for Europe and Southeastern China. Overall low climate suitability was also observed in regions where this cladoceran occurs less frequently in invaded ranges (Fig. 2A).
The most important climatic factor limiting the further expansion of M. macrocopa was the variable bio09/Mean temperature of the driest quarter (Table 1). Bio19/Precipitation of the coldest quarter and bio02/Mean diurnal temperature range were other influential variables. Thus, the probability of occurrence of this species is followed by increases in bio09 but declines with the increase in bio19 and bio02.
The potential distribution map of M. macrocopa in the future is displayed in Fig. 2B. The potential distribution range increased worldwide, with new areas in the American Continent, Asia, Africa, India, and Australia. The suitable areas with suitability ≥ 0.5 for the future climatic conditions were located in the North of Europe, Northeastern Brazil, large portions in the United States, and South Australia. These overall range expansions followed an increase in suitable areas across broad geographic zones except in Europe, which is predicted to decrease in the future scenarios studied (2041–2060) (Fig. 3).
Discussion
Here, we investigated whether the niches of populations of the stress-tolerant cladoceran M. macrocopa remained conserved during the invasion process. We discuss our results focusing on the expansion potential through niche unfilling and further mapped areas more susceptible to global invasion. We found that the climatic niche of M. macrocopa remained broadly stable. Thus, the investigated native and introduced environmental spaces are more similar than random. Also, we found a low degree of expansion (i.e., a new niche in the non-native range) compared to its native niche. However, given the wide variation in environmental conditions where this species currently occur, the niche overlap between American and Palearctic records was low, reflecting their different environmental constraints (Elmoor-Loureiro et al., 2010; Makino et al., 2020; Bhanushali et al., 2021). Thus, during the invasion of the American continent, M. macrocopa retains signatures of its native environmental niche but also indicates differences in environmental space following the introduction, which can be further ascribed to evolutionary processes, dispersal limitations, or invasion history.
In fact, despite niche shifts observed in some taxa (Torres et al., 2018; Macêdo et al., 2021), most invaders do seem to occupy climates similar to those of their source populations (Martínez-Meyer & Peterson, 2006; Petitpierre et al., 2012; Strubbe et al., 2015; Bates et al., 2020). The low but present expansion in M. macrocopa may be due to propagule pressure enhanced by global e-commerce of this species introducing adaptive genetic variation for new areas facilitating colonization of novel environments (Simberloff, 2009). We, therefore, expect propagule pressure to scale the current extent of niche expansion, while decreasing unfilling in the future. On the other hand, hybridization may also impact the evolution of species geographical ranges (Pfennig et al., 2016; Pierce et al., 2017). It is conceivable that hybrids of M. macrocopa are more prone to inhabit significant ecological gradients and occupy a different environmental niche, as demonstrated for other cladoceran species (Wolf & Mort, 1986; Petrusek et al., 2008; Liu et al., 2018), further facilitating invasion (Thornton & Murray, 2014). Also, there is morphological evidence of individuals of an intermediate phenotype from different regions in the American continent, e.g., ventral filaments, in the ephippium (Elías-Gutiérrez & Zamuriano-Claros, 1994; Elmoor-Loureiro et al., 2010; Vignatti et al., 2013). However, we did not directly investigate the niche differentiation of the species by hybridization. Thus, the development of ecological niche models that include biotic interactions should be considered in the future.
Together, the findings above suggest a close link between these records of M. macrocopa, which share environmental niche spaces, corroborating that although closely related, they still may be different taxa. However, apart from the genetic distances between M. macrocopa macrocopa and M. macrocopa americana (Montoliu-Elena et al., 2019; Bhanushali et al., 2021), the very low overlap also brings cues that might be used to test taxonomic hypothesis regarding M. macrocopa macrocopa solely. In addition, the lack of taxonomical identification keys and genetic characterization for many parts of the world (Goulden, 1968) hampers our ability to infer their invasion history accurately.
Currently, there were high portions of the invasive niche of M. macrocopa that remained unfilled or were non-occupied in the non-native range despite being present in the original niche (Simberloff, 2009; Soberón & Arroyo-Pena, 2017). This unfilling indicates environmental non-equilibrium, and that the invasion process of M. macrocopa is incomplete. Niche unfilling can also occur because dispersal is limited, suitable environments are inaccessible, or the initial bottleneck reduces adaptive genetic variation necessary for broad colonization. Nevertheless, the time since introduction can also be correlated with the magnitude of niche filling (Strubbe et al., 2015), suggesting, in this case, a recent invasion of M. macrocopa.
Human activities play an essential role as a vector of new introductions of M. macrocopa—one of the most commonly used cladoceran in standardized laboratory bioassays worldwide (Martínez-Tabche et al., 2000; Iannacone & Alvariño, 2002; Nandini et al., 2004). Following its previous introduction in South America, e.g., in Peru (Valdivia-Villar, 1988), Argentina (Paggi, 1997), and Chile (Iannacone & Alvariño, 2002), this vector is a probable source for its late appearance in Brazil (Elmoor-Loureiro et al., 2010; Rietzler et al., 2014; Eskinazi-Sant’Anna et al., 2020). Hence, as dispersal may not limit its spread in invaded areas, bottleneck and/or biotic resistance are more likely to be the most critical factors determining this high unfilling. M. macrocopa has intense propagule recruitment through clonal or resting egg production (Vignatti et al., 2013; Sirianni, 2017; Nandini & Sarma, 2019), and bottlenecks are common in organisms that recruit novel populations from a single propagule. Explicitly, in this case of M. macrocopa, the selective pressures in cultures intended for aquaculture and live-food production could enhance bottlenecks (Fermin, 1991; Manklinniam et al., 2018). In this case, M. macrocopa population must succumb to the many problems associated with a low genetic variation or adapt to the novel environment relying on plasticity. In addition to the bottleneck effect, the enemy release (the absence of natural competitors or predators; Keane & Crawley, 2002) can also play a role in the success of invasive species, which would enable them to grow and reproduce without these regulatory pressures (Allendorf & Lundquist, 2003). M. macrocopa can often be found in environments with reduced competitive and predation interactions (e.g., low diverse ponds, subjected to abiotic stress, and known to have no fish or invertebrate predators). However, further studies may hypothesize biotic resistance mediated by native aquatic diversity to act against the colonization of available climatic areas for M. macrocopa (Elton, 1958; Levine & D’Antonio, 1999).
Our results also supported the hypothesis of overall geographic expansion following global warming and future predicted hydrological stress, thus forecasting species responses to changing environments in the Anthropocene (Taheri et al., 2021). Moina macrocopa is already common in habitats subject to human pressures, viz. (i) eutrophication, dense populations in nutrient-rich waters such as sewage treatment basins (Vignatti et al., 2013; Padhye & Dumont, 2015), (ii) low oxygen (Paggi, 1997; Elmoor-Loureiro et al., 2010; Vignatti et al., 2013), (iii) salinity variation (5.7 g l−1 to 21.8 g l−1; Vanjare et al., 2010; Vignatti et al., 2013), (iv) rapid recover from resting egg bank affected by heavy metals (Oskina et al., 2019), and (vi) the ability to endure significant temperature variations (minimum of 9.4 and maximum of 26.9 °C; Vignatti et al., 2013), including thermal tolerance above optimum (Engert et al., 2013) when a trade-off between reduced lifespan and increased reproduction occurs in females (Sarma et al., 2005). Such biological attributes of M. macrocopa allow it to establish in many types of waters, including those where extreme environmental conditions limit the presence of zooplankton competitors and where its fitness is reduced, e.g., eutrophic and cyanobacteria dominated (Hansson et al., 2007a, b; Padhye & Dumont, 2015).
The importance of precipitation-related variables would also indicate that this species has essential invasiveness features in unstable environments, e.g., environments subjected to hydrological stress (Alonso, 1996; Vignatti et al., 2013). Accordingly, extreme droughts and rising mean temperature might result in more areas extensively subjected to invasions, at least climatically, by this cladoceran. For example, in some portions of the United States, an increase of 3–9°C in mean annual temperature combined with decreases in precipitation is predicted (Walsh et al., 2014). Also, areas in Australia where high costs with invasive species are reported. Lakes and ponds in Brazilian Pantanal wetlands are currently under severe climate threat. Lakes and ponds in Brazilian Pantanal wetlands that are currently under severe environmental threat. Arid and semi-arid areas in northeastern Brazil, where low precipitation and high-temperature waters tend to expand other planktonic invasive species (Severiano et al., 2022).
Notwithstanding its ability to persist, establish, and expand distribution, M. macrocopa can also favor passive dispersal of epibionts, including parasites becoming vectors of novel introductions of harmful organisms (Xu, 1992; Czeczuga et al., 2008; Vanjare et al., 2010). Also, monitoring ballast water should be considered, especially regarding the high resistance and survival of the resting eggs of M. macrocopa (Alekseev et al., 2010). Further studies using morphology and DNA barcoding can help to foster hypotheses about the invasion process of this potentially invasive cladoceran.
Overall, our findings revealed evidence of conservatism in the M. macrocopa and a high degree of unfilling. Meanwhile, we believe that M. macrocopa is expanding its geographical distribution, currently overlooked, following climate change scenarios. However, the mechanisms by which this species could favor the invasion success are unclear. This potentially invasive species can compete with other congeneric native species common in shallow and temporary environments, such as M. cf. wierzejskii Richard 1895, M. dumonti Kotov, Elías-Gutiérrez & Granado-Ramírez, 2005, and the species complex M. micrura Kurz 1874. We suggest researchers consistently incorporate multivariate analysis of niche into investigations on invasion processes of cladocerans, simultaneously with morphological and molecular information. This integrative approach could more effectively predict future invasions and anticipate detections. In addition, we strongly advocate complete phylogeographical research considering populations in Africa and South America to optimize available information on invasion history and environmental traits in M. macrocopa. In doing so, we would be able to recalibrate our models with updated and novel data to better reflect concurrent changes in species’ realized climatic niche. Ultimately, it could match the proposed interdisciplinarity of Invasion Science.
Data availability
The datasets analyzed during the current study will be made available by the corresponding author on reasonable request.
References
Abell, R., M. L. Thieme, C. Revenga, et al., 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58: 403–414.
Aiello-Lammens, M. E., R. A. Boria, A. Radosavljevic, B. Vilela & R. P. Anderson, 2015. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography 38(5): 541–545.
Alekseev, V., A. Makrushin & J. S. Hwang, 2010. Does the survivorship of activated resting stages in toxic environments provide cues for ballast water treatment? Marine Pollution Bulletin 61: 254–258.
Allendorf, F. W. & L. L. Lundquist, 2003. Population Biology, evolution, and control of invasive species. Conservation Biology 17(1): 24–30.
Alonso, M., A. N. Neretina, L. Sanoamuang, N. Saengphan & A. A. Kotov, 2019. A new species of Moina Baird, 1850 (Cladocera: Moinidae) from Thailand. Zootaxa 4554(1): 199–218.
Alonso, M., 1996. Branchiopoda. Vol. 7. Fauna Iberica. Museo Nacional de Ciencias Naturales. Consejo Superior de Investigaciones Científicas, Madrid: 486 pp.
Araújo, M. B. & M. New, 2007. Ensemble forecasting of species distributions. Trends in Ecology and Evolution. 22(1): 42–47.
Barbet-Massin, M., F. Jiguet, C. H. Albert & W. Thuiller, 2012. Selecting pseudo-absences for species distribution models: how, where and how many? Methods in Ecology and Evolution 3(2): 327–338.
Bates, O. K., S. Ollier & C. Bertelsmeier, 2020. Smaller climatic niche shifts in invasive than non-invasive alien ant species. Nature Communications. https://doi.org/10.1038/s41467-020-19031-1.
Bekker, E. I., D. P. Karabanov, Y. R. Galimov & A. A. Kotov, 2016. DNA barcoding reveals high cryptic diversity in the North Eurasian Moina species (Crustacea: Cladocera). PLoS ONE. https://doi.org/10.1371/journal.pone.0161737.
Bhanushali, S., K. Katti, J. Ramchandani & S. Sen, 2021. A Cost-effective DNA Isolation Strategy from Crustaceans Enables the First Molecular Phylogenetic Identification of Moina macrocopa from India. Genetics of Aquatic Organisms 5: 77–85.
Breiman, L., 2001. Random Forests. Machine Learning 45: 5–32.
Broennimann, O. & A. Guisan, 2008. Predicting current and future biological invasions: both native and invaded ranges matter. Biology Letters 4(5): 585–589.
Broennimann, O., U. A. Treier, H. Müller-Schärer, W. Thuiller, A. T. Peterson & A. Guisan, 2007. Evidence of climatic niche shift during biological invasion. Ecology Letters 10(8): 701–709.
Broennimann, O., M. C. Fitzpatrick, P. B. Pearman, et al., 2012. Measuring ecological niche overlap from occurrence and spatial environmental data: Measuring niche overlap. Global Ecology and Biogeography 21(4): 481–497.
Capinha, C., F. Essl, H. Seebens, D. Moser & H. M. Pereira, 2015. The dispersal of alien species redefines biogeography in the Anthropocene. Science 348(6240): 1248–1251.
Cordier, J. M., R. Loyola, O. Rojas-Soto & J. Nori, 2020. Modeling invasive species risk from established populations: Insights for management and conservation. Perspectives in Ecology and Conservation. https://doi.org/10.1016/j.pecon.2020.06.001.
Czeczuga, B., M. Kozlowska, A. Godlewska & S. C. Velu, 2008. Moina macrocopa (Straus): A Plankton Crustacean as a Vector for Fungus-Like Fish Parasites. Turkish Journal of Zoology 32: 19–26.
Dexter, E. & S. M. Bollens, 2019. Zooplankton invasions in the early 21st century: a global survey of recent studies and recommendations for future research. Hydrobiologia 847: 309–319.
Dormann, C. F., J. Elith, S. Bacher, et al., 2012. Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36(1): 27–46.
Dudgeon, D., 2019. Multiple threats imperil freshwater biodiversity in the Anthropocene. Current Biology 29(19): 960–967.
Dzialowski, A. R., W. J. O’Brien & S. M. Swaffar, 2000. Range expansion and potential dispersal mechanisms of the exotic cladoceran Daphnia lumholtzi. Journal of Plankton Research 22(12): 2205–2223.
Elías-Gutiérrez, M. & R. Zamuriano-Claros, 1994. Primer registro de Moina macrocopa (Daphniiformes: Moinidae) en Bolivia. Revista De Biología 42(1–2): 385.
Elmoor-Loureiro, L. M. A., J. M. Santángelo, P. M. Lopes & R. L. Bozelli, 2010. A new report of Moina macrocopa (Straus, 1820) (Cladocera Anomopoda) in South America. Brazilian Journal Biology 70(1): 225–226.
Elton, C. S., 1958. The Ecology of Invasions by Animals and Plants, Methuen, London:
Engert, A., S. Chakrabarti, N. Saul, M. Bittner, R. Menzel & C. E. W. Steinberg, 2013. Interaction of temperature and an environmental stressor: Moina macrocopa responds with increased body size, increased lifespan, and increased offspring numbers slightly above its temperature optimum. Chemosphere 90(7): 2136–2141.
Eskinazi-Sant’Anna, E. M., G. S. Santos, N. J. S. Alves, L. A. F. Brito & M. G. P. Leite, 2020. The relative importance of regional and local factors in shaping zooplankton diversity in high-altitude tropical shallow lakes. Journal of Freshwater Ecology 35(1): 203–221.
Farias, D. S., L. M. A. Elmoor-Loureiro & C. W. C. Branco, 2017. First record of Moina dumonti Kotov, Elías-Gutiérrez & Granado-Ramírez, 2005 (Branchiopoda: Anomopoda) in Brazil. Check List 13: 2144.
Fermin, A. C., 1991. Freshwater cladoceran Moina macrocopa (Strauss) as an alternative live food for rearing sea bass Lates calcarifer (Bloch) fry. Journal of Applied Ichthyology 7(1): 8–14.
Fick, S. E. & R. J. Hijmans, 2017. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37(12): 4302–4315.
Forró, L., N. M. Korovchinsky, A. A. Kotov & A. Petrusek, 2008. Global diversity of cladocerans (Cladocera; Crustacea) in freshwater. Hydrobiologia 595: 177–184.
Goulden, C. E., 1968. The systematics and evolution of the Moinidae. Transactions of the American Philosophical Society 58(6): 1–101.
Hansson, L., A. Nicolle, J. Brodersen, P. Romare, P. A. Nilsson, C. Brönmark & C. Skov, 2007a. Consequences of fish predation, migration, and juvenile ontogeny on zooplankton spring dynamics. Limnology and Oceanography 52: 696–706.
Hansson, L. A., S. Gustafsson, K. Rengefors & L. Bromark, 2007b. Cyanobacterial chemical warfare affects zooplankton community composition. Freshwater Biology 52: 1290–1301.
Hill, A. P. & E. A. Hadly, 2018. Rethinking “Native” in the Anthropocene. Frontiers in Earth Science. https://doi.org/10.3389/feart.2018.00096.
Iannacone, J. A. & L. Alvariño, 2002. Evaluación del riesgo ambiental del insecticida CARTAP en bioensayos con tres invertebrados. Agricultura Técnica 62(3): 366–374.
Jacobs, M. J. & H. J. MacIsaac, 2007. Fouling of fishing line by the waterflea Cercopagis pengoi: A mechanism of human-mediated dispersal of zooplankton? Hydrobiologia 583(1): 119–126.
Jiménez-Valverde, A., A. T. Peterson, J. Sobéron, J. M. Overton, P. Aragon & J. M. Lobo, 2011. Use of niche models in invasive species risk assessments. Biological Invasions 13: 2785–2797.
Keane, R. M. & M. J. Crawley, 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution 17: 164–169.
Lauzeral, C., F. Leprieur, O. Beauchard, Q. Duron, T. Oberdorff & S. Brosse, 2011. Identifying climatic niche shifts using coarse-grained occurrence data: a test with non-native freshwater fish. Global Ecology and Biogeography 20: 407–414.
Levine, J. M. & C. M. D’Antonio, 1999. Elton revisited: a review of evidence linking diversity and invasibility. Oikos 87: 15–26.
Liu, P., L. Xu, S.-L. Xu, et al., 2018. Species and hybrids in the genus Diaphanosoma Fischer, 1850 (Crustacea: Branchiopoda: Cladocera). Molecular Phylogenetics and Evolution 118: 369–378.
Macêdo, R. L., P. Russo, R. F. Corrêa, O. Rocha, L. N. dos Santos & C. W. C. Branco, 2021. The drifting dinoflagellate Ceratium furcoides (Levander) Langhans 1925: fundamental niche shift during global invasion. Hydrobiologia. https://doi.org/10.1007/s10750-020-04495-5.
Makino, W., R. J. Machida, J. Okitsu & N. Usio, 2020. Underestimated species diversity and hidden habitat preference in Moina (Crustacea, Cladocera) revealed by integrative taxonomy. Hydrobiologia 847: 857–878.
Manklinniam, P., S. Chittapun & S. Maiphae, 2018. Growth and nutritional value of Moina macrocopa (Straus, 1820) fed with Saccharomyces cerevisiae and Phaffia rhodozyma. Crustaceana 91(8): 897–912.
Marquardt, D. W., 1970. Generalized inverses, ridge regression, biased linear estimation, and nonlinear estimation. Technometrics 12: 591–612.
Martínez-Meyer, E. & A. T. Peterson, 2006. Conservatism of ecological niche characteristics in North American plant species over the Pleistocene-to-Recent transition. Journal of Biogeography 33: 1779–1789.
Martínez-tabche, L., L. Gómez-oliván, M. Martínez, C. Castillo & A. Santiago, 2000. Toxicity of nickel in artificial sediment on acetylcholinesterase activity and hemoglobin concentration of the aquatic flea, Moina macrocopa. Journal of Environmental Hydrology 8(4): 1–10.
Meinshausen, M., Z. R. J. Nicholls, J. Lewis, et al., 2020. The shared socio-economic pathway (SSP) greenhouse gas concentrations and their extensions to 2500. Geoscientific Model Development 13: 3571–3605.
Montoliu-Elena, L., M. Elías-Gutiérrez & M. Silva-Briano, 2019. Moina macrocopa (Straus, 1820): a species complex of a common Cladocera, highlighted by morphology and DNA barcodes. Limnetica 38: 253–277.
Müller-Schärer, H., U. Schaffner & T. Steinger, 2004. Evolution in invasive plants: implications for biological control. Trends in Ecology & Evolution 19(8): 417–422.
Naimi, B. & M. B. Araújo, 2016. sdm: a reproducible and extensible R platform for species distribution modelling. Ecography 39: 368–375.
Nandini, S., S. M. Mayeli & S. S. S. Sarma, 2004. Effect of stress on the life table-demography of Moina Macrocopa. Hydrobiologia 526(1): 245–254.
Nandini, S. S. & S. S. S. Sarma, 2019. Reproductive strategies of Moina (Cladocera) in relation to their habitat. Limnetica 38(1): 137–145.
Neretina, A. N., A. G. Kirdyasheva & A. A. Kotov, 2020. Position of Moina wierzejskii Richard, 1895 (Crustacea: Cladocera) within the genus Moina Baird, 1850 in the light of new morphological data. Zootaxa 4820(3): 506–522.
Okolodkov, Y. B., R. Bastida-zavala, A. L. Ibáñez, et al., 2007. Especies acuáticas no indígenas en México. Ciencia y Mar 11(32): 29–67.
Olden, J. D., J. J. Lawler & N. L. Poff, 2008. Machine learning methods without tears: a primer for ecologists. The Quarterly Review of Biology 83: 171–193.
Olden, J. D., E. Whattam & S. A. Wood, 2021. Online auction market places as a global pathway for aquatic invasive species. Hydrobiologia. https://doi.org/10.1007/s10750-020-04407-7.
Oskina, N., T. Lopatina, O. Anishchenko, et al., 2019. High resistance of resting eggs of cladoceran Moina macrocopa to the effect of heavy metals. Bulletin of Environmental Contamination and Toxicology 102: 335–340.
Padhye, S. M. & H. J. Dumont, 2015. Species richness of Cladocera (Crustacea: Branchiopoda) in the Western Ghats of Maharashtra and Goa (India), with biogeographical comments. Journal of Limnology 74(1): 182–191.
Paggi, J. C., 1997. Moina macrocopa (Strauss, 1820) (Branchiopoda, Anomopoda) in South America: Another case of species introduction? Crustaceana 70(8): 886–893.
Palaoro, A. V., M. M. Dalosto, G. C. Costa & S. Santos, 2013. Niche conservatism and the potential for the crayfish Procambarus clarkiito invade South America. Freshwater Biology 58(7): 1379–1391.
Parmesan, C., 2006. Ecological and Evolutionary Responses to Recent Climate Change. Annual Review of Ecology, Evolution, and Systematics 37(1): 637–669.
Pearman, B., A. Guisan, O. Broennimann & C. F. Randin, 2008. Niche dynamics in space and time. Trends Ecol. Evol. 23: 149–158.
Peterson, A. T., 2003. Predicting the geography of species’ invasions via ecological niche modeling. The Quarterly Review of Biology 78: 419–433.
Peterson, A. T. & J. Soberón, 2012. Species Distribution Modeling and Ecological Niche Modeling: Getting the Concepts Right. Natureza & Conservação 10(2): 102–107.
Peterson, A. T. & D. A. Vieglais, 2001. Predicting species invasions using ecological niche modeling. Bioscience 51: 363–371.
Peterson, T., V. Sanchez-Cordero & J. Soberón, 1999. Conservatism of ecological niches in evolutionary time. Science 285: 1265–1267.
Petitpierre, B., C. Kueffer, O. Broennimann, et al., 2012. Climatic niche shifts are rare among terrestrial plant invaders. Science 335: 1344–1348.
Petrusek, A., M. Černý & E. Audenaert, 2004. Large inter-continental differentiation of Moina micrura (Crustacea:Anomopoda): one less cosmopolitan cladoceran? Hydrobiologia 526: 73–81.
Petrusek, A., J. Seda, J. Machácek, S. Ruthova & P. Smilauer, 2008. Daphnia hybridization along ecological gradients in pelagic environments: the potential for the presence of hybrid zones in plankton. Philosophical Transactions of the Royal Society of London. Series b, Biological Sciences 363(1505): 2931–2941.
Pfennig, K. S., A. L. Kelly & A. A. Pierce, 2016. Hybridization as a facilitator of species range expansion. Proceedings of the Royal Society b: Biological Sciences. https://doi.org/10.1098/rspb.2016.1329.
Phillips, S. J., R. P. Anderson & R. E. Schapire, 2006. Maximum entropy modeling of species geographic distributions. Ecological Modelling 190(3–4): 231–259.
Pierce, A. A., R. Gutierrez, A. M. Rice & K. S. Pfennig, 2017. Genetic variation during range expansion: effects of habitat novelty and hybridization. Proceedings of the National Academy of Sciences of the United States of America. https://doi.org/10.1098/rspb.2017.0007.
Pyšek, P., P. E. Hulme, D. Simberloff, et al., 2020. Scientists’ warning on invasive alien species. Biological Reviews of the Cambridge Philosophical Society 95(6): 1511–1534.
R Core Team 2021. R: A language and environment for statistical. computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Reid, A. J., A. K. Carlson, I. F. Creed, et al., 2018. Emerging threats and persistent conservation challenges for freshwater biodiversity. Biological Reviews. https://doi.org/10.1111/brv.12480.
Rietzler, A. C., P. M. Maia-Barbosa, M. M. Ribeiro & R. M. Menendez, 2014. On the first record of the exotic Moina macrocopa (Straus, 1820) in Minas Gerais State, Brazil. Brazilian Journal of Biology 74(2): 518–520.
Ruiz, G. M., P. W. Fofonoff, J. T. Carlton, M. J. Wonham & A. H. Hines, 2000. Invasion of coastal marine communities in North America: Apparent patterns, processes, and biases. Annual Review of Ecology, Evolution, and Systematics 31: 481–531.
Sales, L. P., R. Rebouças & L. F. Toledo, 2021. Native range climate is insufficient to predict anuran invasive potential. Biological Invasions. https://doi.org/10.1007/s10530-021-02528-1.
Sarma, S. S. S., S. Nandini & R. D. Gulati, 2005. Life history strategies of cladocerans: comparisons of tropical and temperate taxa. Hydrobiologia 542: 315–333.
Schoener, T. W., 1970. Nonsynchronous spatial overlap of lizards in patchy habitats. Ecology 51: 408–418.
Severiano, J. S., E. S. Oliveira, D. Lucena-Silva, et al., 2022. Invasion of the dinoflagellate Ceratium furcoides (Levander) Langhans 1925 in South America: record of the pattern of expansion and persistence in tropical reservoirs in Northeastern Brazil. Biological Invasions 24: 217–233.
Simberloff, D., 2009. The role of propagule pressure in biological invasions. Annual Review of Ecology, Evolution, and Systematics 40: 81–102.
Sirianni, K. M., 2017. Differential wind dispersal of cladoceran ephippia in a rock pool metacommunity. Aquatic Ecology 51: 203–218.
Soberón, J. & B. Arroyo-Pena, 2017. Are fundamental niches larger than the realized? Testing a 50-year-old prediction by Hutchinson. PLoS ONE. https://doi.org/10.1371/journal.pone.0175138.
Soeken-Gittinger, L. A., J. A. Stoeckel & J. E. Havel, 2009. Differing effects of suspended sediments on the performance of native and exotic Daphnia. Freshwater Biology 54: 495–504.
Sousa, F. D. R., A. V. Palaoro, L. M. A. Elmoor-Loureiro & A. A. Kotov, 2017. Predicting the invasive potential of the cladoceran Daphnia lumholtzi Sars, 1885 (Crustacea: Cladocera: Daphniidae) in the Neotropics: are generalists threatened and relicts protected by their life-history traits? Journal of Limnology 76: 272–280.
Straus, H. E., 1820. Mémoire sur les Daphnia, de la classe des Crustacés (Seconde Partie). Memoires Du Muséum D’histoire Naturelle 6: 149–162.
Strubbe, D., H. Jackson, J. Groombridge & E. Matthysen, 2015. Invasion success of a global avian invader is explained by within-taxon niche structure and association with humans in the native range. Diversity and Distributions 21(6): 675–685.
Swets, J. A., 1988. Measuring the accuracy of diagnostic systems. Science 240(4857): 1285–1293.
Taheri, S., B. Naimi, C. Rahbek & M. B. Araújo, 2021. Improvements in reports of species redistribution under climate change are required. Science Advances. https://doi.org/10.1126/sciadv.abe1110.
Tebaldi, C., K. Debeire, V. Eyring, et al., 2021. Climate model projections from the Scenario Model Intercomparison Project (ScenarioMIP) of CMIP6. Earth System Dynamics 12(1): 253–293.
Thornton, D. H. & D. L. Murray, 2014. Influence of hybridization on niche shifts in expanding coyote populations. Diversity and Distributions 20(11): 1355–1364.
Tingley, R., M. Vallinoto, F. Sequeira & M. R. Kearney, 2014. Realized niche shift during a global biological invasion. Proceedings of the National Academy of Sciences of the United States of America 111: 10233–10238.
Torres, U., W. Godsoe, H. L. Buckley, M. Parry, A. Lustig & S. P. Worner, 2018. Using niche conservatism information to prioritize hotspots of invasion by non-native freshwater invertebrates in New Zealand. Diversity and Distributions 24(12): 1802–1815.
Valdivia-Villar, R. S., 1988. Checklist of freshwater Cladocera from Perú. Amazoniana 10: 283–297.
Vanjare, A. I., S. M. Padhye & K. Pai, 2010. Zooplankton from a polluted river, Mula (India), with record of Brachionus rubens (Ehrenberg, 1838) epizoic on Moina macrocopa. Opuscula Zoologica 41(1): 89–92.
Vignatti, A. M., G. C. Cabrera & S. A. Echaniz, 2013. Distribution and biological aspects of the introduced species Moina macrocopa (Straus, 1820) (Crustacea, Cladocera) in the semi-arid central region of Argentina. Biota Neotropica 13(3): 86–92.
Walsh, J. R., S. R. Carpenter & M. J. Vander Zanden, 2016. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences of the United States of America 113(15): 4081–4085.
Walsh, J., D. Wuebbles, K. Hayhoe, et al., 2014. In Melillo, J. M., T. Richmond, and G. W. Yohe (eds.) Appendix 3: Climate Science Supplement. Climate Change Impacts in the United States: The Third National Climate Assessment. U.S. Global Change Research Program, 735–789. https://doi.org/10.7930/J0KS6PHH.
Wang, L., Z. Zhang, L. Lin, et al., 2021. Redistribution of the lizardfish Harpadon nehereus in coastal waters of China due to climate change. Hydrobiologia. https://doi.org/10.1007/s10750-021-04682-y.
Warren, D. L., R. E. Glor & M. Turelli, 2008. Environmental niche equivalency versus conservatism: quantitative approaches to niche evolution. Evolution. 62(11): 2868–2883.
Wiens, A., D. Stralberg, D. Jongsomjit, C. A. Howell & M. A. Snyder, 2009. Niches, models, and climate change: Assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences of the United States of America 106(supplement 2): 19729–19736.
Wiens, J. J., D. D. Ackerly, A. P. Allen, et al., 2010. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters 13(10): 1310–1324.
Wolf, H. G. & M. A. Mort, 1986. Inter-specific hybridization underlies phenotypic variability in Daphnia populations. Oecologia 68(4): 507–511.
Xu, Z., 1992. The abundance of epizoic ciliate Epistylis daphniae related to their host Moina macrocopa in an urban stream. Journal of Invertebrate Pathology 60(2): 197–200.
Acknowledgements
This work was funded by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES, doctorate scholarship for RLM). The authors would like to thank the Graduate Course in Ecology and Natural Resources (PPGERN-UFSCar). Last but not least, RLM thanks his amazing dog Darwin who has passed away but whose partnership and company is uniquely reflected in this written work accomplished during the social isolation during the COVID-19 pandemic.
Author information
Authors and Affiliations
Contributions
RLM, LMAE-L, and FDRS: Conceptualization; RLM, LMAE-L, and FDRS: data curation; RLM: formal analysis; OR: funding acquisition; RLM and FDRS: methodology; RLM and OR: writing—original draft; OR, ACR, FDRS, and HJD: writing—review and editing. All authors commented on the previous versions of the manuscript and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflicts of interest regarding this work. We declare that we do not have any commercial or associative interest that represents a conflict of interest with the work submitted.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: José L. Attayde, Renata F. Panosso, Vanessa Becker, Juliana D. Dias & Erik Jeppesen / Advances in the Ecology of Shallow Lakes
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Macêdo, R.L., Sousa, F.D.R., Dumont, H.J. et al. Climate change and niche unfilling tend to favor range expansion of Moina macrocopa Straus 1820, a potentially invasive cladoceran in temporary waters. Hydrobiologia 849, 4015–4027 (2022). https://doi.org/10.1007/s10750-022-04835-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04835-7