Abstract
The objectives of the study were to see if escaped rainbow trout (Oncorhynchus mykiss) spread rapidly or not from fish farms, and to test whether the hydrological conditions in a fjord influence their vertical distribution and importance as vector for the salmon lice (Lepeophtheirus salmonis). Fifty farmed rainbow trout were tagged with acoustic transmitters including depth sensors and released from two of 11 fish farms in the fjord system. In addition, unintentionally escaped rainbow trout were recaptured for analysis of salmon lice and stomach content. Dispersal out of the fjord system was limited. Most fish stayed in the vicinity of and moved between the fish farms but fed primarily on a variety of indigestible items. They moved in the warm relatively fresh surface layer from late spring until early autumn where the risk of being infested with salmon lice was low. They swam gradually deeper and became much more infested with salmon lice as the surface layers cooled and salinity and temperature gradients became less distinct over the course of the winter. The observed post-escapement behavior may challenge the control of the spread of diseases and parasites between neighboring farms and to wild fish, but also increases opportunities for recapture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The rainbow trout (Oncorhynchus mykiss) is one of the most widespread species introduced into Europe for aquaculture (Savini et al., 2010). Global production of rainbow trout has grown exponentially since 1950s, particularly as a result of increased inland production in several European countries and more recently as a consequence of the expansion of mariculture in Norway and Chile (FAO 2011).
The potential environmental effects of cage rearing of rainbow trout have received less attention than those of farmed Atlantic salmon production, primarily because self-sustaining populations of intentionally released or escaped farmed rainbow trout are very rare in Norway (Hesthagen & Sandlund, 2007), as they appear to lack the ability to find suitable spawning habitats (Lindberg et al., 2009) and do not threaten the genetic integrity of the wild Atlantic salmon (Salmo salar) populations. However, rainbow trout farming may increase the risks of spread of diseases and parasites. Rainbow trout host parasites, such as Gyrodactylus salaris (Peeler & Thrush, 2004), and have a high susceptibility to salmon lice (Lepeophtheirus salmonis) (Fast et al., 2002; Gjerde & Saltkjelvik, 2009). They may also carry a number of salmonid diseases (Hodneland et al., 2005; Taksdal et al., 2007; Kristoffersen et al., 2009). Farming and escapes of rainbow trout may therefore contribute to the high infestation rate of sea lice on wild salmonids (Birkeland & Jakobsen, 1997; Finstad et al., 2000; Bjørn et al., 2001; Heuch & Mo, 2001), especially when farms are sited on smolt migration routes (Krkosek et al., 2009).
Few studies have examined the behavior and spread of farmed rainbow trout at sea, but they generally conclude that trout disperse relatively slowly. Bridger et al. (2001) studied the post-release behavior of triploid steelhead trout in Canada and found that most fish were attracted to and remained close to the cages where they had been grown for a long period of time. Skilbrei & Wennevik (2006b) observed that the geographical distribution of gill-net recaptures of escaped rainbow trout agreed well with the localization of the fish farms and with escape events, and Jonsson et al. (1993) concluded that rainbow trout were usually recaptured in the fjord area where they were released. A prolonged stay of escaped rainbow trout in the fjord or coastal areas could increase the risks of negative interaction with wild fish, especially with wild salmonids as Atlantic salmon and sea trout (Salmo trutta) that migrate through or feed in the same areas. The swimming depth of escaped rainbow trout in fjords will influence its probability of being infested with salmon lice. According to laboratory experiments (Johnson & Albright, 1991; Bricknell et al., 2006), the salinity of the upper fresh/brackish water layer in many fjords is lower than the salinity tolerance of salmon. The vertical distribution of the escaped rainbow trout in sea is also important for the design of recapture strategies, but such data have yet to be reported.
The objectives of this study were to study the horizontal and vertical movements of simulated escaped rainbow trout in a fjord system with fish farms to improve our understanding of the interactions between farming of rainbow trout and the environment. It was of interest to study how rapidly they spread in the fjord system and whether their vertical distribution is related to salinity gradients, and to identify their possible role as hosts for salmon lice.
Materials and methods
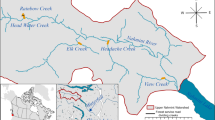
The study was based in the fjord system surrounding the sheltered island of Osterøy in western Norway. A number of rivers that host populations of Atlantic salmon and sea trout enter this basin (Fig. 1). Freshwater run-off produces a brackish water surface layer which make the fjord more suitable for farming of rainbow trout than Atlantic salmon, and all the fish farms in this system have therefore switched to rainbow trout production. Because of a dramatic decline in the numbers of adult Atlantic salmon returning to the River Vosso since the late 1980s, it has been assumed that the survival rate of wild smolts migrating through the fjord system is low. A number of research projects have been and are being carried out in the river and the fjord to identify the causes of this (Barlaup, 2008), and it has been shown that salmon lice may contribute to reduce the survival of released Atlantic salmon smolts (Skilbrei & Wennevik, 2006a).
Study area and locations of receivers (star) in the fjord system surrounding Osterøy, and fish farms with (black circle) or without (black triangle) receivers. The largest rivers draining to the fjord system are shown. Areas referred to in the text as “Northern farms” (NF) and “Southern farms” (SF) are indicated by dashed squares. The remaining area covered by receivers is termed “Elsewhere”
Farmed rainbow trout (0.8–2.5 kg) (Table 1) were randomly selected from net pens at two fish farms and were tagged with V13 acoustic transmitters with depth sensor (V13P-1L-256 coded pingers, 4.3-cm long and 1.2-cm diameter, weight in water 6.6 g, projected battery life 559 days; Vemco Ltd., Nova Scotia, Canada). The fish were anaesthetized with a mix of benzocaine and metomidate. The dose was adjusted so that it took 2–3 min until the fish were calm enough for surgery. The intracoelomic surgical implantation of the transmitters was performed by a trained veterinarian. A 3- to 4-cm-long incision was made 2–3 cm in front of, but 1–2 cm above, the pelvic fin. Terramycin® vet. (Oxytetracycline) was dropped at the tag before inclusion. Tissue adhesive (Histoacryl®) was added to the wound after the three sutures had been closed (Supramid 2/0 polyamide monofilament) and tied with surgeon’s knots. The equipment and needles were sterilized in 70% ethanol. Finally, length and weight were measured and the fish were also tagged with external T-bar anchor tags (Hallprint). The operation took 3–4 min. The fish were first transferred to a tank supplied with running seawater for recovery, and then kept in a net pen for the next 4 or 5 days before being released in late May and late August 2008 (Table 1). The experiment and the tagging procedure were approved by the Norwegian committee for the use of animals in scientific experiments (FDU).
Acoustic receivers (VR2W; Vemco) were attached to 8 of the 11 fish farms in the fjord system (Fig. 1). Receivers were also deployed so that the fish could not leave the fjord system undetected. One receiver covered a narrow strait and two arrays of six receivers were mounted on both sides of the pontoons of a 1.4-km-long floating bridge that crosses the main fjord. A further two receivers were deployed to fill the gap between the southern shore and the first pontoon. Ten additional receivers were attached to floats moored to the bottom and distributed in the fjord system (Fig. 1). All receivers were positioned at depths of 2–3 m, except the one in the strait, at 10 m. Range-testing trials in the Alta Fjord, performed with same type of tags used in the present study, demonstrated that the tags could be registered by receivers at a distance of 600–800 m (Chittenden et al., 2011). However, maximum listening distance may vary considerable, also at the same site (Finstad et al., 2005). In this study, simultaneous recordings at several receivers imply that individuals were occasionally detected at 1–2 km distance.
The following categories were used to describe individual fish whereabouts at a daily basis. “Northern farms”, “Southern farms”, and “Elsewhere” refers to areas described in Fig. 1. It was required that there were >2 recordings of an individual at one or adjacent receivers per day, otherwise it was “Out of range” if it reappeared later, and “Disappeared” if it did not. “Disappeared” also includes two tags of the first release group that appeared to rest at the seabed at a fixed depth and position for many months (these fish may have died, lost their tag or been captured and gutted). The tags of “Captured” fish were recovered by fishers using gill-nets or anglers, and a fish was categorized as “Out of the fjord” if its last known position was by (the western side of) the bridge crossing the entrance to the Osterøy fjord basin. To calculate the estimated position of a fish that was not within the range of a receiver at 12:00 h on selected dates, it was assumed that it had swum at constant speed from the previous to the next receiver, taking the shortest possible route. Occasional recordings (1–5/day) of two individuals on the receiver 1.4 km away from release site 2 were used to verify that they were most probably staying in the vicinity of release site 2 during parts of the period when the receiver at release site 2 was missing.
Several receivers were lost and replaced during the experiment. Data were therefore lost from release site 2 and the receiver adjacent to it toward the north-east from Aug 28 to Oct 15, 2008, and from Oct 15, 2008 to Jan 15, 2009 from the two fish farms in the middle part of the “Southern farms” area (Fig. 1). The number of receivers was reduced on Dec 3, 2008 when the “Elsewhere” receivers were removed, except for those covering the strait and the bridge and one in the “Southern Farm” area. Daily temperature and salinity measurements at 1, 4, and 8 m depth were available from the northernmost of the fish farms with receivers.
Escaped farmed rainbow trout were captured during 2009 to quantify salmon lice infestation and stomach content. The authorities received two escape reports from fish farmers in the fjord in autumn 2008, and many reports on recaptures from anglers and fishers mainly in the area covered by “Southern farms” (Fig. 1) during autumn/early winter 2008/2009, including filed reports on the catch of 598 rainbow trout by 9 fishers in the annual gill-net fishery that targets escapees from Oct 1 to 28 Feb (personal communication G. Walle, Department of Environmental Affairs, Hordaland County). Three samples of the escaped rainbow trout were collected. On March 23 and on May 1, 2009 escaped fish were captured by trolling, and from 22 to 25 June 2009 rainbow trout were caught in a bag-net within the area “Southern farms”.
According to the monthly reports from the fish farmers in the fjord system to the authorities reporting the numbers of lice on samples of farmed fish and treatments against salmon lice, farmed fish were not treated against salmon lice between May 2008 and June 2009. One fish farm reported 0.01 adult female lice per fish, and 0.2 lice per fish of (the other) movable stages of lice (males lice and younger stages) in March 2009. Another farm found a mean of 0.1 lice per fish of movable stages in January, February, and March 2009. All other reports from the fish farms showed zero numbers of salmon lice from May 2008 to June 2009 (personal communication Lise Torkildsen, the Norwegian Food Safety Authority).
Results
The rainbow trout that were released from one of the “Southern farms” in late May 2008 resided primarily within the “Southern farm” area during the summer and early autumn, except for the fish (~20%), that moved out of the fjord during June 2008 (Figs. 2, 3, and 4). The number of fish that stayed in the vicinity of the release site for at least 1 h/day decreased from 6 to 9 individuals (30–45% of released fish), to 4–8 (20–40%) and thereafter to 2–7 (10–35%) fish per day during the periods 1–10, 11–20, and 21–75 days post-release, respectively. Several individuals (n = 5, 25%) also visited the bridge in June 2008. They typically moved to the bridge during 1 day, stayed in that area or moved to the most remote receivers for the next 2–5 days before using a day or two to travel the >20-km-long distance back to their “home” area. While ~50% of the fish stayed primarily in the proximity of one single receiver for weeks, the other half moved more and were frequently visiting 4–7 of the 7 receivers in the ~10-km-long “Southern farms” area during a single day. Twenty percent (n = 5) were reported recaptured by October 2008 in the fjord within the area covered by the receivers, and 40% (n = 8) disappeared (Fig. 2).
Whereabouts of release group 1 from release on May 27 to Oct 15, 2008 (upper panel) and of release group 2 from release on Aug 24 to Dec 3, 2008 (lower panel). See “Materials and methods” for detailed definition of categories. Bars are smoothed by presenting the mean of the every 3 days
A Percentage of the number of release group 1 fish recorded daily at each receiver from May 27 to Oct 15, 2008. Sizes of the pie charts vary with the percentages of fish, from 0.07 to 29.65%. B Percentage of the number of release group 2 fish recorded daily at each receiver from Aug 24 to Oct 15, 2008. Sizes of the pie charts vary with the percentages of fish, from 0.25 to 14.66%
The fish released in late August from one of the “Northern farms” spread rapidly to most farms in the fjord during the first week (Fig. 2). The number of fish that stayed in the vicinity of the release site for at least 1 h/day decreased from 12 to 4 individuals (from 40 to 13%) from day 1 to 5 post-release. Approximately one half of the fish stayed among the “Northern farms” and adjacent receivers (most of the “Elsewhere” group), while the other half moved quickly down to the “Southern farms” (Figs. 2, 3). Fish (13 out of 30) also moved between “Northern” and “Southern farms” 2–4 times during autumn. As a consequence of this the average distance from the fish to their release site were clearly higher for the second than for the first release group; 7–10 versus 3–5 km (Fig. 4). Thirty-seven percent left the fjord system during the autumn, and 30% were recaptured (Fig. 2). One of these had left the fjord and was captured 20 km west of the bridge and one other was angles in River Lone. The rest (n = 11) was caught within the area covered by the receivers. The loss of receivers (see “Materials and methods”) increased the “Out of range” category of the fish moving in the “Northern farms” area from late August to mid-October (Fig. 2).
The 10 individuals (3 of release 1 and 7 of release 2) that stayed in the fjord in early December 2008 were still present in early April 2009. However, all of the seven fish of the second release moved out of the fjord during 5 weeks from April 6 to May 8, 2009. The three remaining fish disappeared during May and June 2009.
The swimming depth of the fish changed with the annual temperature cycle. Generally, the fish stayed very close to the surface in low salinity water during summer, but moved gradually deeper as temperature decreased during autumn and winter (Fig. 5), especially when the temperature dropped below 5°C (Fig. 6). Maximum swimming depths were reached during February–March. Mean depth was 7–12 m, but the standard deviation was high (Fig. 5) because the individuals were spread over depths from 4 to 30 m. In March, the temperatures were relatively uniform at depths from 1 to 8 m (4–6°C), and the brackish layer had started to build up again after being less distinct, with surface salinities above 20°C in early February. The fish also responded to high temperatures during the summer by increasing their swimming depth by 2–4 m when the temperature of the surface layer rose above ~16 to 18°C (Figs. 5, 6).
Swimming depth (Y) versus temperature at 1-m depth. Mean of every 3 days from May 28 to Aug 25, 2008 (white circle, Release 1) and from Aug 25 to April 28, 2009 (white square, Release groups 1 and 2). The exponential function y = 0.674 + exp(2.775 − 0.234x) has been fitted to the data (F = 91, R 2 = 0.58, P < 0.0001)
The behavior and swimming depth of one individual of the second release differed from the rest of the fish. It stayed almost permanently within the listening range of the receiver at one of the northern fish farms from late August until it was recaptured in late November 2008, and moved frequently between surface and 15–25 m depth, sometimes many times per day. The net pens at the fish farm were ~25- to 30-m deep.
The infestation rate of salmon lice on escaped rainbow trout dropped clearly during the spring. In late March 2009 there were 15 lice per fish, in early May there were 9 lice in average, and in late June 2009 the numbers were close to zero (Table 2). Adult lice dominated over younger stages on all three sample dates. High numbers of chalimus and preadult stages were found only on two individuals recaptured in late March.
Few recaptured escaped rainbow trouts had food pellets in their stomachs (n = 3, 4.4%) (Table 2), and 22% of the stomachs were empty (n = 15). Apart from small blue mussels (Mytilus edulis) that were frequently found in the June sample (in 18 stomachs, 60% of sample) and in two other individuals, the stomach contents consisted of a variety of items that had probably been picked up from the surface; leaves, flowers, sticks and pieces of wood, pine needles, kelp and seaweed, cigarettes filters, and plastic-like waste.
Discussion
This study confirms that released rainbow may remain in the vicinity of the release site for several months (Jonsson et al., 1993; Bridger et al., 2001; Rikardsen & Sandring, 2006). It also showed that the fish did not necessarily stay close to the fish farm from which they had been released, but stayed in the vicinity of or moved between other fish farms in the fjord.
Unexpectedly, the recaptured escaped fish did not feed on surplus pellets to any significant extent. The behavioral study seems to confirm this, as only one of the 31 tagged fish stayed permanently in the vicinity of a fish farm for a long period of time, and behaved as if it may have had responded to the feeding in the fish farm. This finding is in contrast to the observation that escaped Atlantic salmon that remain in the vicinity of the fish farm may feed largely on pellets (Olsen & Skilbrei, 2010). The reasons why this food source was not utilized are not known. It may be that rainbow trout do not compete effectively with the marine fish, mostly saithe (Pollachius virens), which aggregate beneath the net pens in great numbers (Dempster et al., 2009). The lack of proper food items in the stomachs of the recaptures agrees well with the findings of Rikardsen & Sandring (2006), that escaped rainbow trout fed on indigestible items, such as seaweed and small pieces of wood that are similar in shape to the commercial pellets to which they were accustomed, but contrasts with a study of escaped rainbow trout in Chile that fed on a variety of wild prey (Soto et al., 2001). However, the age of that fish at escape was unknown in the study of Soto et al. (2001) and may have contributed to this discrepancy. Rikardsen & Sandring (2006) suggested that rainbow trout that escape as adults, unlike postsmolts, have difficulties in learning to find wild prey. One reason for this may be that European rainbow trout have been domesticated for many generations, having been introduced in Europe around 100 years ago.
Why then do the majority of the escaped farmed rainbow trout remain close to the fish farms if they do not feed there? Migratory behavior may simply be poorly developed in the strains used for mariculture or in fish of this size. For example, the tendency of escaped farmed Atlantic salmon to move rapidly toward open sea is highly dependent on the developmental stage of the fish at the time of escape (Skilbrei, 2010). However, the movements out of the fjord of 20% of the fish in June 2008, and most of the remaining fish in April/May 2009, are reminiscent of a study with Atlantic salmon that showed that migratory behavior may not develop exclusively at the smolt stage, but also in adults during the spring (Hansen & Jonsson, 1989). On the other hand, the return of the fish that visited the bridge to their “home” area (21 km distance), and their long residency in the home area is similar to the observations of Bridger et al. (2001), who suggested that the return of trout to the rearing site implies that some level of orientation exists, based on cues or imprinting established while the fish are growing at the site. Escaped fish may also be attracted to familiar smells and sounds from the fish farms. Migrating Atlantic salmon use odors from conspecifics for local orientation (Johannesson, 1987), and it has been suggested that acoustic conditioning of rainbow trout (Abbott, 1972) could be used to aggregate escapees to improve recapture rates (Tlusty et al., 2008).
This study provides novel information regarding the relationship between the vertical distribution of escaped farmed rainbow trout and the risk of becoming infested with salmon lice. The “escapees” moved close to the relatively fresh surface layer as long as the temperature there was higher than in deeper waters, and above 5°C. The escaped rainbow trout may prefer the physical conditions (light intensity, temperature, and salinity) in the upper water column during spring and summer, but this behavior may also have been learned when the fish were reared in the net pens. The food is distributed close to the surface in the net pens. The finding of a variety of floating items in their stomachs (see above) show that they continue to search for food close to the surface after they escape. At lower temperatures, and with less clear salinity and temperature gradients in late winter, they were much more spread throughout the water column. Low temperature reduces the capacity for osmotic regulation in rainbow trout (Finstad et al., 1988) and may be one reason for the downward movement to warmer water. The surface salinity of 6–12 during the spring and summer is a hostile environment for salmon lice, so fish that stay in this layer are probably relatively well protected against lice. Tank experiments (Heuch, 1995; Bricknell et al., 2006) suggest that the success of the copepodids decreases with salinity, but that they are probably capable of infesting salmonids at salinities of ~18 to 25. Both the salinity profile and the vertical distribution of the escaped rainbow trout therefore suggest that they could have been infested with salmon lice during the winter, an interpretation that was confirmed by the infestation rates of all stages of salmon lice on escaped rainbow trout recaptured in late March. The reduced infestation rate in May and very low infestation rate in June also seem to fit with the expectation that salmon lice parasitism is less likely after the escaped fish return to the fresher surface layer during spring. These relationships imply that the hydrological conditions in the fjord become important for the interactions among escaped rainbow trout, sea lice, and wild salmonids in the fjord. The risks of infestation with salmon lice would then be higher during winter and early spring when much of the precipitation falls as snow, lower during the melting of snow in the drainage basins during the spring, and variable in summer, depending on rainfall and how the operation of the hydropower plants in the major rivers influences the freshwater run-off. The Atlantic salmon smolts leave the rivers and migrate through the fjord from late April until June (Skilbrei et al., 2010b). The risk of being ingested with salmon lice may then be higher for the fish that migrate early before the buildup of a surface layer with low salinity. The production of salmon lice nauplius in the fjord system throughout the year have potentially a larger influence on sea trout, which feed in the fjord and are present there from the winter (old individuals) to late autumn.
In contrast to the high infestation of salmon lice on the escaped rainbow trout in late winter 2009, there were almost no findings of adult female salmon lice on the farmed rainbow trout in the fjord system and no need for the farmers to delouse the fish in 2008 and 2009. One reason may be that the farmed fish move in the upper water column during feeding, and that the salinity there was low enough during the winter 2008/2009 to reduce the settlement and development success of the salmon louse. In accordance with the model that predicts that the significance of escaped farm fish as a vector for salmon lice may be high when the louse burden in the fish farms is low (Heuch & Mo, 2001), the presence of escaped rainbow trout may well have contributed considerably to the total production of salmon lice eggs in the fjord system during late winter/early spring.
Movements of escaped fish could help to explain some of the unknown factors in the models employed in assessment of the risks of transfer of pathogens between fish farms (Skilbrei et al., 2010a), especially for horizontally transferred pathogens as pancreatic disease (Kristoffersen et al., 2009). The movements of the escaped farmed rainbow trout between fish farms and their long stay in the fjord imply that the escaped fish may, at worst, become a reservoir for pathogens once they have been introduced into the fjord system which may challenge the disease management and control.
The reported recapture rate (35%) and the slow dispersal of fish out of the fjord system imply that a significant portion of rainbow trout that escape from these sites could be recaptured. This is supported by catch statistics from the autumn gill-net fishery for escaped salmonids, which revealed that the catch of rainbow trout per unit effort was high after escapes and largely within this fjord basin compared to adjacent areas (Skilbrei & Wennevik, 2006b). As many fish moved away from their home farm and spread to or moved between other fish farms, the fishing effort must be geographically distributed to cover all local fish farms if it is to be effective. This behavior also implies that the common practice of using gill-nets attached to the fish farm or set in the immediate neighborhood of it as an early warning of escape incidents can be misleading, and that additional information, such as genetic profiles may be necessary if we wish to identify the farm of origin of unreported escapes of rainbow trout (Glover, 2008). The distribution of the rainbow trout close to the surface during summer, which is beneficial because traditional fishing gears for salmonids can be used, is similar to the vertical distribution of simulated escaped Atlantic salmon. The differences are that Atlantic salmon may dive well below the maximum depth observed in this study and do not avoid cold surface water during the winter (Skilbrei et al., 2009). The increased individual vertical range during the winter may well influence the catchability of escaped rainbow trout because the fish occupy a larger volume and will be less available in traditional fishing gears for salmonids like floating gill-nets and bag-nets that operate close to the surface.
In summary, although the escaped farmed rainbow trout did not appear to feed on surplus food pellets from the fish farms, they did remain in the vicinity of the farms for a lengthy period of time and dispersed slowly out of the fjord system. This suggests that the risk of transfer of pathogens between fish farm was increased, possibly also to wild fish in the fjord. The importance of escaped rainbow trout as vectors of salmon lice appear to depend on whether, and at what time, there is a relatively fresh surface layer warmer than 5°C in the fjord. Above this temperature they stay close to the surface, while at lower temperatures they move deeper into higher salinity water where the risk of being infested with salmon lice increases. The fish were available for recapture for weeks and even months within the fjord system, but an effective recapture strategy would require the fished area to cover the neighborhood of all fish farms.
References
Abbott, R. R., 1972. Induced aggregation of pond-reared Rainbow-trout (Salmo gairdneri) through acoustic conditioning. Transactions of the American Fisheries Society 101: 35–43.
Barlaup, B. T., 2008. Nå eller aldri for Vossolaksen—anbefalte tiltak med bakgrunn i bestandsutvikling og trusselfaktorer. DN-utredning 2008-9. Available at http://www.dirnat.no/content/632/ (in Norwegian).
Birkeland, K. & P. Jakobsen, 1997. Salmon lice, Lepeophtheirus salmonis, infestation as a causal agent of premature return to rivers and estuaries by sea trout, Salmo trutta, juveniles. Environmental Biology of Fishes 49: 129–137.
Bjørn, P. A., B. Finstad & R. Kristoffersen, 2001. Salmon lice infection of wild sea trout and Arctic char in marine and freshwaters: the effects of salmon farms. Aquaculture Research 32: 947–962.
Bricknell, I. R., S. J. Dalesman, B. O’Shea, C. C. Pert & A. J. M. Luntz, 2006. Effect of environmental salinity on sea lice Lepeophtheirus salmonis settlement success. Diseases of Aquatic Organisms 71: 201–212.
Bridger, C. J., R. K. Booth, R. S. McKinley & D. A. Scruton, 2001. Site fidelity and dispersal patterns of domestic triploid steelhead trout (Oncorhynchus mykiss Walbaum) released to the wild. ICES Journal of Marine Science 58: 510–516.
Chittenden, C. M., A. H. Rikardsen, O. T. Skilbrei, J. G. Davidsen, E. Halttunen, J. Skardhamar & R. S. McKinley, 2011. An effective method for the recapture of escaped farmed salmon. Aquaculture Environment Interactions 1: 215–224.
Dempster, T., I. Uglem, P. Sanchez-Jerez, D. Fernandez-Jover, J. Bayle-Sempere, R. Nilsen & P. A. Bjørn, 2009. Coastal salmon farms attract large and persistent aggregations of wild fish: an ecosystem effect. Marine Ecology-Progress Series 385: 1–14.
Finstad, B., M. Staurnes & O. B. Reite, 1988. Effect of low temperature on sea-water tolerance in rainbow trout, Salmo gairdneri. Aquaculture 72: 319–328.
Finstad, B., P. A. Bjørn, A. Grimnes & N. A. Hvidsten, 2000. Laboratory and field investigations of salmon lice [Lepeophtheirus salmonis (Krøyer)] infestation on Atlantic salmon (Salmo salar L.) post-smolts. Aquaculture Research 31: 795–803.
Finstad, B., F. Økland, E. B. Thorstad, P. A. Bjørn & R. S. McKinley, 2005. Migration of hatchery-reared Atlantic salmon and wild anadromous brown trout post-smolts in a Norwegian fjord system. Journal of Fish Biology 66: 86–96.
FAO 2011. FishStat Plus. FAO, Rome, Italy.
Fast, M. D., N. W. Ross, A. Mustafa, D. E. Sims, S. C. Johnson, G. A. Conboy, D. J. Speare, G. Johnson & J. F. Burka, 2002. Susceptibility of rainbow trout Oncorhynchus mykiss, Atlantic salmon Salmo salar and coho salmon Oncorhynchus kisutch to experimental infection with sea lice Lepeophtheirus salmonis. Diseases of Aquatic Organisms 52: 57–68.
Gjerde, B. & B. Saltkjelvik, 2009. Susceptibility of Atlantic salmon and rainbow trout to the salmon lice Lepeophtheirus salmonis. Aquaculture 291: 31–34.
Glover, K., 2008. Genetic characterisation of farmed rainbow trout in Norway: intra- and inter-strain variation reveals potential for identification of escapees. BMC Genetics 9: 87.
Hansen, L. P. & B. Jonsson, 1989. Salmon ranching experiments in the river Imsa: effect of timing of Atlantic salmon (Salmo salar) smolt migration on survival to adults. Aquaculture 82: 367–373.
Hesthagen, T. & O. T. Sandlund, 2007. Non-native freshwater fishes in Norway: history, consequences and perspectives. Journal of Fish Biology 71: 173–183.
Heuch, P. A., 1995. Experimental evidence for aggregation of salmon lice copepodids (Lepeophtheirus salmonis) in step salinity gradients. Journal of the Marine Biological Association of the United Kingdom 75: 927–939.
Heuch, P. A. & T. A. Mo, 2001. A model of salmon louse production in Norway: effects of increasing salmon production and public management measures. Diseases of Aquatic Organisms 45: 145–152.
Hodneland, K., A. Bratland, K. E. Christie, C. Endresen & A. Nylund, 2005. New subtype of salmonid alphavirus (SAV), Togaviridae, from Atlantic salmon Salmo salar and rainbow trout Oncorhynchus mykiss in Norway. Diseases of Aquatic Organisms 66: 113–120.
Johannesson, B., 1987. Observations related to the homing instinct of Atlantic salmon (Salmo salar L.). Aquaculture 64: 339–341.
Johnson, S. C. & L. J. Albright, 1991. Development, growth, and survival of Lepeophtheirus salmonis (Copepoda, Caligidae) under laboratory conditions. Journal of the Marine Biological Association of the United Kingdom 71: 425–436.
Jonsson, N., B. Jonsson, L. P. Hansen & P. Aas, 1993. Potential for sea ranching rainbow trout, Oncorhynchus mykiss (Walbaum): evidence from trials in two Norwegian fjords. Aquaculture and Fisheries Management 24: 653–661.
Krkosek, M., A. Morton, J. P. Volpe & M. A. Lewis, 2009. Sea lice and salmon population dynamics: effects of exposure time for migratory fish. Proceedings of the Royal Society B 276: 2819–2828.
Kristoffersen, A. B., H. Viljugrein, R. T. Kongtorp, E. Brun & P. A. Jansen, 2009. Risk factors for pancreas disease (PD) outbreaks in farmed Atlantic salmon and rainbow trout in Norway during 2003–2007. Preventive Veterinary Medicine 90: 127–136.
Lindberg, M., P. Rivinoja, L. O. Eriksson & A. Alanärä, 2009. Post-release and pre-spawning behaviour of simulated escaped adult rainbow trout Oncorhynhus mykiss in Lake Övre Fryken, Sweden. Journal of Fish Biology 74: 691–698.
Olsen, R. E. & O. T. Skilbrei, 2010. Feeding preference of recaptured Atlantic salmon Salmo salar following simulated escape from fish pens during autumn. Aquaculture Environment Interactions 1: 167–174.
Peeler, E. J. & M. A. Thrush, 2004. Qualitative analysis of the risk of introducing Gyrodactylus salaris into the United Kingdom. Diseases of Aquatic Organisms 62: 103–113.
Rikardsen, A. H. & S. Sandring, 2006. Diet and size-selective feeding by escaped hatchery rainbow trout Oncorhynchus mykiss (Walbaum). ICES Journal of Marine Science 63: 460–465.
Savini, D., A. Occhipinti-Ambrogi, A. Marchini, E. Tricarico, F. Gherardi, S. Olenin & S. Gollasch, 2010. The top 27 animal alien species introduced into Europe for aquaculture and related activities. Journal of Applied Ichthyology 26: 1–7.
Skilbrei, O. T., 2010. Reduced migratory performance of farmed Atlantic salmon post-smolts from a simulated escape during autumn. Aquaculture Environment Interactions 1: 117–125.
Skilbrei, O. T. & V. Wennevik, 2006a. Survival and growth of sea-ranched Atlantic salmon, Salmo salar L., treated against sea lice before release. ICES Journal of Marine Science 63: 1317–1325.
Skilbrei, O. T. & V. Wennevik, 2006b. The use of catch statistics to monitor the abundance of escaped farmed Atlantic salmon and rainbow trout in the sea. ICES Journal of Marine Science 63: 1190–1200.
Skilbrei, O. T., J. C. Holst, L. Asplin & M. Holm, 2009. Vertical movements of “escaped” farmed Atlantic salmon (Salmo salar L.)—a simulation study in a western Norwegian fjord. ICES Journal of Marine Science 66: 278–288.
Skilbrei, O. T., J. C. Holst, L. Asplin & S. Mortensen, 2010a. Horizontal movements of simulated escaped farmed Atlantic salmon (Salmo salar L.) in a western Norwegian fjord. ICES Journal of Marine Science 67: 1206–1215.
Skilbrei, O. T., V. Wennevik, G. Dahle, B. Barlaup & T. Wiers, 2010b. Delayed smolt migration of stocked Atlantic salmon parr (Salmo salar). Fisheries Management and Ecology 17: 493–500.
Soto, D., F. Jara & C. Moreno, 2001. Escaped salmon in the inner seas, southern Chile: facing ecological and social conflicts. Ecological Applications 11: 1750–1762.
Taksdal, T., A. B. Olsen, I. Bjerkås, M. J. Hjortaas, B. H. Dannevig, D. A. Graham & M. F. McLoughlin, 2007. Pancreas disease in farmed Atlantic salmon, Salmo salar L., and rainbow trout, Oncorhynchus mykiss (Walbaum), in Norway. Journal of Fish Diseases 30: 545–558.
Tlusty, M. F., J. Andrew, K. Baldwin & T. M. Bradley, 2008. Acoustic conditioning for recall/recapture of escaped Atlantic salmon and rainbow trout. Aquaculture 274: 57–64.
Acknowledgments
I thank Sjøtroll Havbruk AS and Lerøy Fossen AS and their fish farm staff for their contributions and cooperation, and Gunnar Bakke and Vidar Wennevik for assistance during field work. field work. I am also grateful to Håkon R. Sæbø for doing the surgery, Hugh M. Allen for his comments to the manuscript and two anonymous reviewers for their helpful advices. Financial support was provided by the Institute of Marine Research.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Koen Martens
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Skilbrei, O.T. The importance of escaped farmed rainbow trout (Oncorhynchus mykiss) as a vector for the salmon louse (Lepeophtheirus salmonis) depends on the hydrological conditions in the fjord. Hydrobiologia 686, 287–297 (2012). https://doi.org/10.1007/s10750-012-1028-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-012-1028-x