Abstract
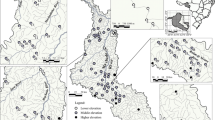
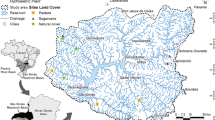
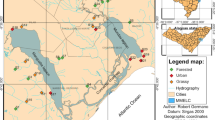
Fish are important in the structuring of other communities and may have large effects on the functioning of aquatic ecosystems. The structure of fish communities, in turn, seems to differ with climate. We compared the characteristics of fish assemblages in lowland streams located in two contrasting climates (cold-temperate Europe and subtropical South America) by use of published and unpublished data on streams of similar depth, width, and slope (n total = 91 streams). We also selected a subset of seven comparable little-affected streams in the two contrasting climates: temperate (Denmark, 55°–57°N, Dk) and subtropical (Uruguay, 30°–35°S, Uy) and compared the fish community structures in relation to environmental characteristics. We then analysed a series of potential explanatory factors behind the patterns observed, in particular the effect of ambient temperature, by comparing temperature-corrected community metabolism. Significantly higher species richness, higher densities, lower biomass, smaller mean body size, and lower mean weight of fish were observed for the subtropical streams than for the temperate streams, both in the literature review and in the subset of streams. Several characteristics of fish assemblages in streams may be explained by direct and indirect effects of temperature. Accordingly, fish in subtropical systems had a temperature-corrected community metabolism I m−2 equal to that of fish in temperate systems, indicating that temperature, besides historical factors, is an important driver of different size structures. Our findings concur with differences previously found in littoral areas of shallow lakes, suggesting that these patterns are not restricted to running waters. Our results elucidate how fish community structure might be affected by increases in temperature triggered by climate warming.





Similar content being viewed by others
References
Abell, R., M. L. Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. C. Balderas, W. Bussing, M. L. J. Stiassny, P. Skelton, G. R. Allen, P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, J. V. Higgins, T. J. Heibel, E. Wikramanayake, D. Olson, H. L. López, R. E. Reis, J. G. Lundberg, M. H. P. Sabaj & P. Petry, 2008. Freshwater ecoregions of the world: a new map of biogeographic units for freshwater biodiversity conservation. BioScience 58: 403–414.
Agostinho, A. A. & T. Penczak, 1995. Populations and production of fish in two small tributaries of the Paraná River, Paraná, Brazil. Hydrobiologia 312: 153–166.
Allan, J. D., 1995. Stream Ecology: Structure and Function of Running Waters. Chapman & Hall, New York.
Allen, A. P., J. H. Brown & J. F. Gillooly, 2002. Global biodiversity, biochemical kinetics, and the energetic-equivalence rule. Science 297: 1545–1548.
Allen, A. P., J. F. Gillooly, V. M. Savage & J. H. Brown, 2006. Kinetic effects of temperature on rates of genetic divergence and speciation. Proceedings of the National Academy of Sciences of the United States of America 103: 9130–9135.
Almirón, A. E., M. L. García, R. C. Menni, L. C. Protoginio & L. C. Solari, 2000. Fish ecology of a seasonal lowland stream in temperate South America. Marine and Freshwater Research 51: 265–274.
Andersen, B. G. & H. W. Borns, 1994. The Ice Age World. Scandinavian University Press, Oslo.
APHA., 1985. Standard Methods for the Examination of Water and Wastewater. APHA-AWWA-WPCF, Washington.
Atkinson, D., 1994. Temperature and organism size – a biological law for ectotherms. Advances in Ecological Research 25: 1–58.
Atkinson, D., B. J. Ciotti & D. J. S. Montagnes, 2003. Protists decrease in size linearly with temperature: ca. 2.5% °C−1. Proceedings of the Royal Society of London Series B-Biological Sciences 270: 2605–2611.
Becker, F. G., S. de Carvalho & S. M. Hartz, 2008. Life-history of the South American darter, Characidium pterostictum (Crenuchidae): evidence for small scale spatial variation in a piedmont stream. Neotropical Ichthyology 6: 591–598.
Bergmann, C., 1847. Uber die Verhältnisse der Wärmeökonomie der Tiere zu ihrer Grösse. Gottinger Studien 1: 595–708.
Blackburn, T. M., K. J. Gaston & N. Lorder, 1999. Geographic gradients in body size: a clarification of Bergmann’s rule. Diversity and Distributions 5: 165–174.
Brown, J. H., J. F. Gilloly, A. P. Allen, V. M. Savage & G. B. West, 2004. Toward a metabolic theory of ecology. Ecology 85: 1771–1789.
Brown, J. H., A. P. Allen & J. F. Gilloly, 2007. The metabolic theory of ecology and the role of body size in marine and freshwater ecosystems. In Hildrew, A., D. Rafaelli & R. Edmons-Brown (eds), Body Size: The Structure and Function of Aquatic Ecosystems. Cambridge University Press, Cambridge: 1–15.
da Silva Abes, S. S. & A. A. Agostinho, 2001. Spatial patterns in fish distributions and structure of the ichthyocenosis in the Água Nanci stream, upper Paraná River basin, Brazil. Hydrobiologia 445: 217–227.
Damuth, J., 1981. Population density and body size in mammals. Nature 290: 699–700.
Damuth, J., 2007. A macroevolutionary explanation of energy equivalence in the scaling of body size and population density. American Naturalist 169: 621–631.
Daufresne, M. & P. Boët, 2007. Climate change impacts on structure and diversity of fish communities in rivers. Global Change Biology 13: 2467–2478.
Daufresne, M., K. Lengfellner & U. Sommer, 2009. Global warming benefits the small in aquatic ecosystems. Proceedings of the National Academy of Sciences of the United States of America 106: 12788–12793.
Diepernik, C. 2003. Fisk og naturkvalitet i vandløb. WaterFrame, Ry: 57 pp (in Danish).
Eichbaum-Esteves, K. & J. Lobón-Cerviá, 2001. Composition and trophic structure of a fish community of a clear water Atlantic rainforest stream in southeastern Brazil. Environmental Biology of Fishes 62: 429–440.
Facey, D. E. & G. D. Grossman, 1990. The metabolic cost of maintaining position for four North American stream fishes: effects of season and velocity. Physiological Zoology 63: 757–776.
Fernandez, E. M., R. A. Ferriz, C. A. Bentos & G. R. López, 2008. Icthyofauna of two streams in the high basin of the Samborombón River, Buenos Aires province, Argentina. Revista del Museo Argentino de Ciencias Naturales 10: 147–154.
Ferreira, K. M., 2007. Biology and ecomorphology of stream fishes from the rio Mogi-Guaçu basin, Southeastern Brazil. Neotropical Ichthyology 5: 311–326.
Forster, J., A. G. Hirst & D. Atkinson, 2011. How do organisms change size with changing temperature? The importance of reproductive method and ontogenetic timing. Functional Ecology 25: 1024–1031.
Friberg, N., A. Baattrup-Pedersen, M. Lauge Pedersen & J. Skriver, 2005. The new Danish stream monitoring programme (NOVANA) preparing monitoring activities for the water framework directive era. Environmental Monitoring and Assessment 111: 2–42.
Gillooly, J. F. & S. I. Dodson, 2000. Latitudinal patterns in the size distribution and seasonal dynamics of new world, freshwater cladocerans. Limnology and Oceanography 45: 22–30.
Gillooly, J. F., J. H. Brown, G. B. West, V. M. Savage & E. L. Charnov, 2001. Effects of size and temperature on metabolic rate. Science 293: 2248–2251.
González-Bergonzoni, I., M. Meerhoff, T. A. Davidson, F. Teixeira-de Mello, A. Baattrup-Pedersen & E. Jeppesen. Meta-analysis shows a consistent and strong latitudinal pattern in fish omnivory across ecosystems. Ecosystems (accepted)
Gordon, N. D., T. A. McMahon & B. L. Finlayson, 1992. Stream Hydrology: An Introduction for Ecologists. John Wiley & Sons Ltd., Chichester, England.
Goyenola, G., C. Iglesias, N. Mazzeo & E. Jeppesen, 2011. Analysis of the reproductive strategy of Jenynsia multidentata (Cyprinodontiformes, Anablepidae) with focus on sexual differences in growth, size, and abundance. Hydrobiologia 673: 245–257.
Griffiths, D., 2006. Pattern and process in the ecological biogeography of European freshwater fish. Journal of Animal Ecology 75: 734–751.
Griffiths, D., 2011. Body size distributions in North American freshwater fish: large-scale factors. Global Ecology and Biogeography doi:10.1111/j.1466-8238.2011.00680.x.
Hillebrand, H., 2004. On the generality of the latitudinal diversity gradient. American Naturalist 163: 192–211.
Jackson, D. A., P. R. Peres-Neto & J. D. Olden, 2001. What controls who is where in freshwater fish communities – the roles of biotic, abiotic, and spatial factors. Canadian Journal of Fisheries and Aquatic Sciences 58: 157–170.
Jeppesen, E., M. Meerhoff, K. Holmgren, I. González-Bergonzoni, F. Teixeira-de Mello, S. A. J. Declerck, L. De Meester, M. Søndergaard, T. L. Lauridsen, R. Bjerring, J. M. Conde-Porcuna, N. Mazzeo, C. Iglesias, M. Reizenstein, H. J. Malmquist, Z. W. Liu, D. Balayla & X. Lazzaro, 2010. Impacts of climate warming on lake fish community structure and potential ecosystem effects. Hydrobiologia 646: 73–90.
Junge, C. O. & J. Libosvárský, 1965. Effects of size selectivity on population estimates based on successive removals with electrical fishing gear. Zoologické Listy 14: 171–178.
Kingsolver, J. G. & R. B. Huey, 2008. Size, temperature, and fitness: three rules. Evolutionary Ecology Research 10: 251–268.
Mahon, R. & E. K. Balon, 1985. Fish production in warm water streams in Poland and Ontario. Canadian Journal of Fisheries and Aquatic Sciences 42: 1211–1215.
Mazzoni, R. & J. Lobón-Cerviá, 2000. Longitudinal structure, density and production rates of a neotropical stream fish assemblage: the river Ubatiba to the Serra do Mar, southeast Brazil. Ecography 23: 588–602.
McKee, D., D. Atkinson, S. Collings, J. Eaton, I. Harvey, T. Heyes, K. Hatton, D. Wilson & B. Moss, 2002. Macrozooplankter responses to simulated climate warming in experimental freshwater microcosms. Freshwater Biology 47: 1557–1570.
McNab, B. K., 1999. On the comparative ecological and evolutionary significance of total and mass-specific rates of metabolism. Physiological and Biochemical Zoology 72: 642–644.
Meerhoff, M., J. M. Clemente, F. Teixeira-de Mello, C. Iglesias, A. R. Pedersen & E. Jeppesen, 2007. Can warm climate-related structure of littoral predator assemblies weaken clear water state in shallow lakes? Global Change Biology 13: 1888–1897.
Meerhoff, M., F. Teixeira-de Mello, C. Kruk, C. Alonso, I. González-Bergonzoni, J. P. Pacheco, M. Arim, M. Beklioğlu, S. Brucet, G. Goyenola, C. Iglesias, G. Lacerot, N. Mazzeo, S. Kosten, & E. Jeppesen. Environmental warming in shallow lakes: a review of effects on community structure as evidenced from space-for-time substitution approach. Advances in Ecological Research (accepted).
Meissner, K. & T. Muotka, 2006. The role of trout in stream food webs: integrating evidence from field surveys and experiments. Journal of Animal Ecology 75: 421–433.
Mortensen, E., 1977. Population, survival, growth and production of trout Salmo trutta in a small Danish stream. Oikos 28: 9–15.
Nee, S., A. F. Read & P. H. Harvey, 1991. The relationship between abundance and body size in British birds. Nature 351: 312–313.
Oberdorff, T., J. F. Guégan & B. Hugueny, 1995. Global scale patterns of fish species richness in rivers. Ecography 18: 345–352.
Ohlberger, J., G. Staaks & F. Hölker, 2007. Estimating the active metabolic rate (AMR) in fish based on tail beat frequency (TBF) and body mass. Journal of Experimental Zoology 307: 296–300.
Pedersen, M. L. & A. Baattrup-Pedersen, 2003. Ecological Monitoring in Streams and Riparian Areas in NOVANA 2004–2009. National Environmental Research Institute. Technical Guidelines from NERI no. 21 [available on internet at http://tekniske-anvisninger.dmu.dk] (in Danish).
Penczak, T., 1981. Ecological fish production in two small lowland rivers in Poland. Oecologia 48: 107–111.
Poff, N. L. & J. V. Ward, 1989. Implications of streamflow variability and predictability for lotic community structure: a regional analysis of streamflow patterns. Canadian Journal of Fisheries and Aquatic Sciences 46: 1805–1817.
Pringle, C. M. & T. Hamazaki, 1998. The role of omnivory in a neotropical stream: separating diurnal and nocturnal effects. Ecology 79: 269–280.
Randall, R. G., J. R. M. Kelso & C. K. Minns, 1995. Fish production in freshwaters: are rivers more productive than lakes? -Canadian Journal of Fisheries and Aquatic Sciences 52: 631–643.
Schmid, P. E., M. Tokeshi & J. M. Schmid-Araya, 2000. Relation between population density and body size in stream communities. Science 289: 1557–1560.
Schlosser, I. J., 1990. Environmental variation, life history attributes, and community structure in stream fishes: implications for environmental management and assessment. Environmental Management 14: 621–628.
Seber, G. A. F. & E. D. Le Cren, 1967. Estimating population parameters from catches large relative to the population. Journal of Animal Ecology 36: 631–643.
Silva, A., L. Quintana, M. Galeano & P. Errandonea, 2003. Biogeography and breeding in Gymnotiformes from Uruguay. Environmental Biology of Fishes 66: 329–338.
Spranza, J. J. & E. H. Stanley, 2000. Condition, growth, and reproductive styles of fishes exposed to different environmental regimes in a prairie drainage. Environmental Biology of Fishes 59: 99–109.
Svendsen, L. M. & B. Norup, 2005. NOVANA. National Monitoring and Assessment Programme for the Aquatic and Terrestrial Environments. Programme Description – Part 1. NERI Technical Report no. 532. National Environmental Research Institute, Denmark.
Teixeira-de Mello, F., M. Meerhoff, Z. Pekcan-Hekim & E. Jeppesen, 2009. Substantial differences in littoral fish community structure and dynamics in subtropical and temperate shallow lakes. Freshwater Biology 54: 1202–1215.
Teixeira-de Mello, F., I. Gonzalez-Bergonzoni & M. Loureiro, 2011. Peces de agua dulce de Uruguay. PPR-MGAP: 188.
Tonn, W. M., 1990. Climate change and fish communities: a conceptual framework. Transactions of the American Fisheries Society 119: 337–352.
Valderrama, J. C., 1981. The simultaneous analysis of total N and total P in natural waters. Marine Chemistry 10: 109–122.
Van Deventer, J. S. & W. S. Platts, 1985. A Computer Software System for Entering, Managing, and Analyzing Fish Capture Data from Streams. Research Note INT-352, Intermountain Forest & Range Research Station. U.S.D.A. Forest Service, Ogden, UT.
Vanni, M. J., 2002. Nutrient cycling by animals in freshwater ecosystems. Annual Review of Ecology and Systematics 33: 341–370.
Vari, R. P., & L. R. Malabarba, 1998. Neotropical icthyologhy: an overview. In Malabarba, L. R., R. E. Reis, R. P. Vari, Z. M. S. Lucena & C. A. S. Lucena (eds), Phylogeny and classification of neotropical fishes. Edipucrs, Porto Alegre, p. 11.
Walter, R., W. R. Hill, M. G. Ryon & E. M. Schilling, 1995. Light limitation in a stream ecosystem: responses by primary producers and consumers. Ecology 76: 1297–1309.
Winemiller, K. O. & K. A. Rose, 1992. Patterns of life history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences 49: 2196–2218.
Woodward, G., D. M. Perkins & L. E. Brown, 2010. Climate change and freshwater ecosystems: impacts across multiple levels of organization. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 365: 2093–2106.
Wootton, R. J., 1990. Ecology of Teleost Fishes. I. Chapman and Hall, New York, NY.
Zar, J. H., 1999. Biostatistical Analysis. New Jersey, Prentice Hall.
Acknowledgments
We are grateful to Anne Mette Poulsen for manuscript editing and Juana Jacobsen for graphical assistance. We deeply acknowledge the field assistance of César Fagúndez and Tito Olivera, the laboratory assistance of Soledad García, Soledad Marroni, Juan Pablo Pacheco, and Mariana Vianna, the logistic support and generosity of the Teixeira-de Mello family and the landowners and land workers in “Republic” of Tacuarembó, Uruguay, and the comments of Matías Arim. FTM, MM, and NM received support from the SNI (Agencia Nacional de Investigación e Innovación, ANII, Uruguay). FTM is supported by a PhD scholarship from the SNB (ANII, Uruguay) and PEDECIBA, and MM by ANII-FCE (2009-2749) and the national award L’Oréal-UNESCO for Women in Science Uruguay (with support of DICyT). In Denmark, support was provided by EU EUROLIMPACS and EU REFRESH, the Research Council for Nature and Universe (272-08-0406), the STF project CRES, and FNU (16-7745). This paper received the Harald Sioli Prize award at the XIII Brazilian Congress of Limnology 2011 (Natal, Brazil).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Sidinei Magela Thomaz
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Teixeira-de Mello, F., Meerhoff, M., Baattrup-Pedersen, A. et al. Community structure of fish in lowland streams differ substantially between subtropical and temperate climates. Hydrobiologia 684, 143–160 (2012). https://doi.org/10.1007/s10750-011-0979-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-011-0979-7