Abstract
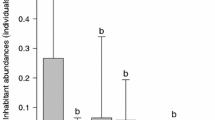
New habitat on proliferating marine construction may increase jellyfish polyp populations, and thereby increase jellyfish populations worldwide. In this investigation, we examined planula settlement and polyp immigration rates of the scyphozoan Aurelia labiata Chamisso & Eysenhardt, 1821 on six common dock-building materials. The planulae and polyps preferred plastics (expanded polystyrenes, low and high density polyethylene) to rubber and treated wood when choosing habitat on man-made surfaces. Substrate surface texture and the presence/absence of anti-fouling chemicals are discussed as possible causes for these substrate preferences. This study illustrates the potential effects of different man-made structures on jellyfish populations, and provides useful information to coastal managers and port authorities for reduction of biofouling and jellyfish bloom effects.




Similar content being viewed by others
References
Arai, M. N., this volume. Are podocysts important to the formation of scyphozoan blooms? Hydrobiologia.
Brewer, R. H., 1978. Larval settlement behaviour in the jellyfish Aurelia aurita (Linnaeus) (Scyphozoa: Semaeostomae). Estuaries 1: 121–122.
Brodeur, R. D., C. E. Mills, J. E. Overland, G. E. Walters & J. G. Schumacher, 1999. Evidence for a substantial increase in gelatinous zooplankton in the Bering Sea, with possible links to climate change. Fisheries Oceanography 8: 296–306.
Brodeur, R. D., H. Sugisaki & G. L. Hunt, Jr., 2002. Increases in jellyfish biomass in the Bering Sea: implications for the ecosystem. Marine Ecology Progress Series 233: 89–103.
Brooks, K. M., 1996. Evaluating the environmental risks associated with the use of chromated copper arsenate-treated wood products in aquatic environments. Estuaries 19: 296–305.
Brooks, K. M., 1999. Recommendations to the National Marine Fisheries Service for the use of CCA-C, ACZA and creosote treated wood products in aquatic environments where threatened or endangered species occur. Western Wood Preservers Institute, Vancouver, Washington.
Buss, L. W., 1990. Competition within and between encrusting clonal invertebrates. Trends in Ecology & Evolution 5: 352–356.
Eckman, J. E., 1990. A model for passive settlement by planktonic larvae onto bottoms of differing roughness. Limnology and Oceanography 35: 887–901.
Estes, J. A., M. T. Tinker, T. M. Williams & D. F. Doak, 1998. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science 282: 473–476.
Fisheries and Oceans Canada, 2002. Saskatchewan fact sheet 3: what you should know about fish habitat and building materials. http://www.dfo-mpo.gc.ca/canwaters-eauxcan/infocentre/guidelines-conseils/factsheets-feuillets/manitoba/pdf/fact3e.pdf.
Gong, A. J., 2002. Allocations to clonal replication in a marine scyphozoan (Aurelia). Science & Engineering 62: 3516–3635.
Gröndahl, F., 1988. Interactions between polyps of Aurelia aurita and planktonic larvae of scyphozoans: an experimental study. Marine Ecology Progress Series 45: 87–93.
Gröndahl, F., 1989. Evidence of gregarious settlement of planula larvae of the scyphozoan Aurelia aurita: an experimental study. Marine Ecology Progress Series 56: 119–125.
Grosberg, R. K., 1981. Competitive ability influences habitat choice in marine invertebrates. Nature 290: 700–702.
Hernroth, L. & F. Gröndahl, 1985a. On the biology of Aurelia aurita (L.). 3. Predation by Coryphella verrucosa (Gastropoda, Opisthobranchia), a major factor regulating the development of Aurelia populations in the Gullmar Fjord, Western Sweden. Ophelia 24: 37–45.
Hernroth, L. & F. Gröndahl, 1985b. On the biology of Aurelia aurita (L.). 2. Major factors regulating the occurrence of ephyrae and young medusae in the Gullmar Fjord, Western Sweden. Bulletin of Marine Science 37: 567–576.
Holst, S. & G. Jarms, 2007. Substrate choice and settlement preferences of planula larvae of five Scyphozoa (Cnidaria) from German Bight, North Sea. Marine Biology 151: 863–871.
Ishii, H. & U. Båmstedt, 1998. Food regulation of growth and maturation in a natural population of Aurelia aurita (L.). Journal of Plankton Research 20: 805–816.
Ishii, H. & K. Katsukoshi, this volume. Distribution and changes in abundance of Aurelia aurita polyps on a pier in the innermost part of Tokyo Bay.
Ishii, H. & F. Tanaka, 2001. Food and feeding of Aurelia aurita in Tokyo Bay with an analysis of stomach contents and a measurement of digestion times. Hydrobiologia 451: 311–320.
Keen, S. L., 1987. Recruitment of Aurelia aurita (Cnidaria: Scyphozoa) larvae is position-dependent, and independent of conspecific density, within a settling surface. Marine Ecology Progress Series 38: 151–160.
Lindahl, O. & L. Hernroth, 1983. Phyto-zooplankton community in coastal waters of western Sweden – an ecosystem off balance. Marine Ecology Progress Series 10: 119–126.
Lo, W.-T., J. E. Purcell, J.-J. Hung, H.-M. Su & P.-K. Hsu, 2008. Enhancement of jellyfish (Aurelia aurita) populations by extensive aquaculture rafts in a coastal lagoon in Taiwan. ICES Journal of Marine Science 65.
Lotan, A., R. Ben-Hillel & Y. Loya, 1992. Life cycle of Rhopilema nomadica: a new immigrant scyphomedusan in the Mediterranean. Marine Biology 122: 237–242.
Lucas, C. H., 2001. Reproduction and life history strategies of the common jellyfish, Aurelia aurita, in relation to its ambient environment. Hydrobiologia 451: 229–246.
Lucas, C. H., A. G. Hirst & J. A. Williams, 1997. Plankton dynamics and Aurelia aurita production from two contrasting ecosystems: causes and consequences. Estuarine Coastal and Shelf Science 45: 209–219.
Mills, C. E., 1995. Medusae, siphonophores and ctenophores as planktivorous predators in changing global ecosystems. ICES Journal of Marine Science 52: 575–581.
Mills, C. E., 2001. Jellyfish blooms: are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451: 55–68.
Miyake, H., J. Hashimoto, M. Chikuchishin & T. Miura, 2004. Scyphopolyps of Sanderia malayensis and Aurelia aurita attached to the tubes of vestimentiferan tube worm, Lamellibrachia satsuma, at submarine fumaroles in Kagoshima Bay. Marine Biotechnology 6: S174–S178.
Miyake, H., K. Iwao & Y. Kakinuma, 1997. Life history and environment of Aurelia aurita. South Pacific Studies 17: 273–285.
Miyake, H., M. Terazaki & Y. Kakinuma, 2002. On the polyps of the common jellyfish Aurelia aurita in Kagoshima Bay. Journal of Oceanography 58: 451–459.
Möller, H., 1980. Scyphomedusa as predators and food competitors of larval fish. Meeresforschung 28: 90–100.
Olsson, P., E. Granéli, P. Carlsson & P. Abreu, 1992. Structuring of a post spring phytoplankton community by manipulation of trophic interactions. Journal of Experimental Marine Biology and Ecology 158: 249–266.
Papathanassiou, E., P. Panayotidis & K. Anagnlstaki, 1987. Notes on the biology and ecology of the jellyfish Aurelia aurita Lam. In Elefsis Bay (Saronikos Gulf, Greece). Pubblicazioni della Stazione Zoologica di Napoli I. Marine Ecology 8: 49–58.
Pitt, K. A., 2000. Life history and settlement preferences of the edible jellyfish Catostylus mosaicus (Scyphozoa: Rhizostomeae). Marine Biology 136: 269–279.
Purcell, J. E., 2003. Predation on zooplankton by large jellyfish, Aurelia labiata, Cyanea capillata and Aequorea aequorea in Prince William Sound, Alaska. Marine Ecology Progress Series 246: 137–152.
Purcell, J. E., 2005. Climate effects on formation of jellyfish and ctenophore blooms. Journal of the Marine Biology Association of the U.K. 85: 1–16.
Purcell, J. E., 2007. Environmental effects on asexual reproduction rates of the scyphozoan, Aurelia labiata. Marine Ecology Progress Series 348: 183–196.
Purcell, J. E. & M. N. Arai, 2001. Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451: 27–44.
Purcell, J. E. & M. V. Sturdevant, 2001. Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Marine Ecology Progress Series 210: 67–83.
Purcell, J. E., S.-I. Uye & W.-T. Lo, 2007. Anthropogenic causes of jellyfish blooms and direct consequences for humans: a review. Marine Ecology Progress Series 350: 153–174.
Robinson, C. H., 1970. Density regulation in populations of scyphistomae. M.A. Thesis. University of California, Davis, USA.
Russell, F. S., 1970. The medusae of the British Isles. II Pelagic Scyphozoa with a supplement to the first volume on Hydromedusae. Cambridge University Press, London: 284 pp.
Schneider, G. & G. Behrends, 1994. Population dynamics and the trophic roles of Aurelia aurita medusae in the Kiel Bight and western Baltic. ICES Journal of Marine Science 51: 359–367.
Schneider, G. & G. Behrends, 1998. Top–down control in a neritic plankton system by Aurelia aurita medusae–a summary. Ophelia 48: 71–82.
Smayda, T., 1993. Experimental manipulations of phytoplankton + zooplankton + ctenophore communities, and foodweb roles of the ctenophore Mnemiopsis leidyi. ICES cm 1993/L: 68: 13 pp.
UNEP (United Nations Environmental Programme), 1991. Jellyfish blooms in the Mediterranean, Proceedings of II Workshop on Jellyfish in the Mediterranean Sea, Mediterranean Action Plan Technical Reports Series: 47.
Uye, S. & H. Shimauchi, 2005. Population biomass, feeding, respiration and growth rates, and carbon budget of the scyphomedusa Aurelia aurita in the Inland Sea of Japan. Journal of Plankton Research 27: 237–248.
Uye, S. & U. Ueta, 2004. Recent increase of jellyfish populations and their nuisance to fisheries in the inland Sea of Japan. Bulletin of the Japanese Society of Fisheries Oceanography 68: 9–19.
Wantanabe, T. & H. Ishii, 2001. In situ estimation of ephyrae liberated from polyps of Aurelia aurita using settling plates in Tokyo Bay, Japan. Hydrobiologia 451: 247–258.
Xian, W., B. Kang & R. Liu, 2005. Jellyfish blooms in the Yangtze estuary. Science 307: 41.
Acknowledgments
We would like to thank the director and staff of Shannon Point Marine Center for supplying the facilities, equipment, and support to make this study possible, The Charles and June Ross Foundation and the Biology department of Western Washington University for their financial support, Brian Williams of the Washington Department of Fish and Wildlife for his technical expertise, Mr. Dundee, owner of the Cornet Bay Marina for allowing us to conduct experiments at his facilities, Dr. Brian Bingham for advise concerning experimental design, and Nathan Schwarck for his support and friendship throughout. This research was funded in part by National Science Foundation ADVANCE Fellows Award OCE-0137419.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: K. A. Pitt & J. E. Purcell
Jellyfish Blooms: Causes, Consequences, and Recent Advances
Rights and permissions
About this article
Cite this article
Hoover, R.A., Purcell, J.E. Substrate preferences of scyphozoan Aurelia labiata polyps among common dock-building materials. Hydrobiologia 616, 259–267 (2009). https://doi.org/10.1007/s10750-008-9595-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-008-9595-6