Abstract
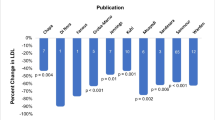
Coronary allograft vasculopathy (CAV) continues to afflict a high number of heart transplant (HT) recipients, and elevated LDL is a key risk factor. Many patients cannot tolerate statin medications after HT; however, data for alternative agents remains scarce. To address this key evidence gap, we evaluated the safety and efficacy of the PCSK9i after HT through systematic review and meta-analysis. We searched Medline, Cochrane Central, and Scopus from the earliest date through July 15th, 2021. Citations were included if they were a report of PCSK9i use in adults after HT and reported an outcome of interest. Outcomes included change in LDL cholesterol from baseline, incidence of adverse events, and evidence of CAV. Changes from baseline and outcome incidences were pooled using contemporary random-effects model methodologies. A total of six studies including 97 patients were included. Over a mean follow-up of 13 months (range 3–21), PCSK9i use lowered LDL by 82.61 mg/dL (95% CI − 119.15 to − 46.07; I2 = 82%) from baseline. Serious adverse drug reactions were rarely reported, and none was attributable to the PCSK9i therapy. Four studies reported stable calcineurin inhibitor levels during PCSK9i initiation. One study reported outcomes in 33 patients with serial coronary angiography and intravascular ultrasound, and PCSK9i were associated with stable coronary plaque thickness and lumen area. One study reported on immunologic safety, showing no DSA development within 1 month of therapy. Preliminary data suggest that long-term PCSK9i therapy is safe, significantly lowers LDL, and may attenuate CAV after HT. Additional study on larger cohorts is warranted to confirm these findings.

Similar content being viewed by others
References
Chih S, Chong AY, Mielniczuk LM, Bhatt DL, Beanlands RS (2016) Allograft vasculopathy: the achilles’ heel of heart transplantation. J Am Coll Cardiol 68:80–91
Costanzo MR, Dipchand A, Starling R et al (2010) International society of heart and lung transplantation guidelines. The international society of heart and lung transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant 29:914–956
Harris J, Teuteberg J, Shullo M (2018) Optimal low-density lipoprotein concentration for cardiac allograft vasculopathy prevention. Clin Transplant 32:e13248
Karatasakis A, Danek BA, Karacsonyi J et al (2017) Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta-analysis of 35 randomized controlled trials. J Am Heart Assoc 6:e006910
Page MJ, McKenzie JE, Bossuyt PM et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed 27 Dec 2021
Follmann D, Elliott P, Suh I, Cutler J (1992) Variance imputation for overviews of clinical trials with continuous response. J Clin Epidemiol 45(7):769–773
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 19(14):135
Knapp G, Hartung J (2003) Improved tests for a random effects meta-regression with a single covariate. Stat Med 22(17):2693–2710
IntHout J, Ioannidis JP, Rovers MM, Goeman JJ (2016) Plea for routinely presenting prediction intervals in meta-analysis. BMJ Open 6(7):e010247
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560
Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP (2011) Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343:d4002
Sammour Y, Dezorzi C, Austin BA et al (2021) PCSK9 inhibitors in heart transplant patients: safety, efficacy, and angiographic correlates. J Card Fail 27(7):812–815
Jennings DL, Jackson R, Farr M (2020) PCSK9 inhibitor use in heart transplant recipients: a case series and review of the literature. Transplantation 104:e38–e39
Di Nora C, Sponga S, Livi U (2019) Safety and efficacy of PCSK9 inhibitor treatment in heart transplant patients. Transplantation 103:e58
Groba-Marco MDV, Castillo-García SD, Barge-Caballero G, Barge-Caballero E, Couto-Mallón D, Crespo-Leiro MG (2019) Treatment of hypercholesterolemia with PCSK9 inhibitors in heart transplant recipients. First Experience in Spain. Rev Esp Cardiol (Engl Ed) 72:1084–1086
Kühl M, Binner C, Jozwiak J et al (2019) Treatment of hypercholesterolaemia with PCSK9 inhibitors in patients after cardiac transplantation. In: Feng Y-M (ed) PLOS ONE. vol 14, pp e0210373
Moayedi Y, Kozuszko S, Knowles JW et al (2019) Safety and efficacy of PCSK9 inhibitors after heart transplantation. Can J Cardiol 35:104.e1-104.e3
Sandesara PB, Dhindsa D, Hirsh B, Jokhadar M, Cole RT, Sperling LS (2019) PCSK9 inhibition in patients with heart transplantation: a case series. J Clin Lipidol 13:721–724
Uyanik-Uenal K, Stoegerer-Lanzenberger M, Auersperg A et al (2019) Treatment of therapy-resistant hyperlipidaemia after heart transplant with PCSK9 inhibitors. J Heart Lung Transplant 38:S213–S214
Bikak A, Berei T, Nissen-Boryczka K et al (2019) Immunologic response to PCSK9 inhibitors in orthotopic heart transplant recipients: a case series. J Card Fail 25:S27
McCollum J, Clary JM, Guglin ME et al (2019) Utilization of PCSK-9 inhibitors in statin intolerant cardiac transplant patients. J Card Fail 26:S106–S107
Vallakati A, Reddy S, Dunlap ME, Taylor DO (2016) Impact of statin use after heart transplantation. Circ Heart Fail 9:e003265
Broch K, Gude E, Karason K et al (2020) Cholesterol lowering with EVOLocumab to prevent cardiac allograft Vasculopathy in De-novo heart transplant recipients: design of the randomized controlled EVOLVD trial. Clin Transplant 34:e13984
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Jennings is a speaker/consultant for Abiomed, Merck, Novartis, La Jolla, and AstraZeneca.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jennings, D.L., Sultan, L., Mingov, J. et al. PCSK9 inhibitors safely and effectively lower LDL after heart transplantation: a systematic review and meta-analysis. Heart Fail Rev 28, 149–156 (2023). https://doi.org/10.1007/s10741-022-10255-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-022-10255-5