Abstract
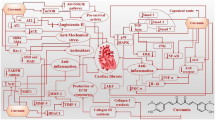
Cardiac fibrosis is one of the most common pathological conditions caused by different heart diseases, including myocardial infarction and diabetic cardiomyopathy. Cardiovascular disease is one of the major causes of mortality worldwide. Cardiac fibrosis is caused by different processes, including inflammatory reactions and oxidative stress. The process of fibrosis begins by changing the balance between production and destruction of extracellular matrix components and stimulating the proliferation and differentiation of cardiac fibroblasts. Many studies have focused on finding drugs with less adverse effects for the treatment of cardiovascular disease. Some studies show that nutraceuticals are effective in preventing and treating diseases, including cardiovascular disease, and that they can reduce the risk. However, big clinical studies to prove the therapeutic properties of all these substances and their adverse effects are lacking so far. Therefore, in this review, we tried to summarize the knowledge on pathways and mechanisms of several nutraceuticals which have shown their usefulness in the prevention of cardiac fibrosis.
Similar content being viewed by others
Data availability
Not applicable.
References
Gu HP, Lin S, Xu M, Yu HY, du XJ, Zhang YY, Yuan G, Gao W (2012) Up-regulating relaxin expression by G-quadruplex interactive ligand to achieve antifibrotic action. Endocrinology 153:3692–3700
Yu C-M, Tipoe GL, Lai KW-H, Lau C-P (2001) Effects of combination of angiotensin-converting enzyme inhibitor and angiotensin receptor antagonist on inflammatory cellular infiltration and myocardial interstitial fibrosis after acute myocardial infarction. J Am Coll Cardiol 38:1207–1215
Querejeta R, López B, González A et al (2004) Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation 110:1263–1268
Li L, Zhao Q, Kong W (2018) Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69:490–506
Park S, Nguyen NB, Pezhouman A, Ardehali R (2019) Cardiac fibrosis: potential therapeutic targets. Transl Res 209:121–137
Ma ZG, Yuan YP, Wu HM, Zhang X, Tang QZ (2018) Cardiac fibrosis: new insights into the pathogenesis. Int J Biol Sci 14:1645–1657
Aronson JK (2017) Defining 'nutraceuticals': neither nutritious nor pharmaceutical. Br J Clin Pharmacol 83:8–19
Rajapakse T, Pringsheim T (2016) Nutraceuticals in migraine: a summary of existing guidelines for use. Headache 56:808–816
Santini A, Cammarata SM, Capone G, Ianaro A, Tenore GC, Pani L, Novellino E (2018) Nutraceuticals: opening the debate for a regulatory framework. Br J Clin Pharmacol 84:659–672
Cicero AFG, Colletti A, Bajraktari G et al (2017) Lipid-lowering nutraceuticals in clinical practice: position paper from an international lipid expert panel. Nutr Rev 75:731–767
Cicero AFG, Colletti A, von Haehling S, et al. (2020) Nutraceutical support in heart failure: a position paper of the International Lipid Expert Panel (ILEP). Nutr Res Rev: 1-25
Imenshahidi M, Hosseinzadeh H (2016) Berberis Vulgaris and berberine: an update review. Phytother Res 30:1745–1764
Cicero AF, Baggioni A (2016) Berberine and its role in chronic disease. Adv Exp Med Biol 928:27–45
Birdsall TC (1997) Berberine: therapeutic potential of alkaloid found in several medicinal plants. Altern Med Rev 2:94–103
Tabeshpour J, Imenshahidi M, Hosseinzadeh H (2017) A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran J Basic Med Sci 20:557–568
Imanshahidi M, Hosseinzadeh H (2008) Pharmacological and therapeutic effects of Berberis vulgaris and its active constituent, berberine. Phytother Res 22:999–1012
Banach M, Patti AM, Giglio RV, Cicero AFG, Atanasov AG, Bajraktari G, Bruckert E, Descamps O, Djuric DM, Ezhov M, Fras Z, von Haehling S, Katsiki N, Langlois M, Latkovskis G, Mancini GBJ, Mikhailidis DP, Mitchenko O, Moriarty PM, Muntner P, Nikolic D, Panagiotakos DB, Paragh G, Paulweber B, Pella D, Pitsavos C, Reiner Ž, Rosano GMC, Rosenson RS, Rysz J, Sahebkar A, Serban MC, Vinereanu D, Vrablík M, Watts GF, Wong ND, Rizzo M, International Lipid Expert Panel (ILEP) (2018) The role of nutraceuticals in statin intolerant patients. J Am Coll Cardiol 72:96–118
Zhao HP, Hong Y, Xie JD, Xie XR, Wang J, Fan JB (2007) Effect of berberine on left ventricular remodeling in renovascular hypertensive rats. Yao Xue Xue Bao 42:336–341
Borrell-Pagès M, Romero JC, Juan-Babot O, Badimon L (2011) Wnt pathway activation, cell migration, and lipid uptake is regulated by low-density lipoprotein receptor-related protein 5 in human macrophages. Eur Heart J 32:2841–2850
Badimon L, Casaní L, Camino-Lopez S, Juan-Babot O, Borrell-Pages M (2019) GSK3β inhibition and canonical Wnt signaling in mice hearts after myocardial ischemic damage. PLoS One 14
Borrell-Pages M, Vilahur G, Romero J, Casaní L, Bejar M, Badimon L (2016) LRP5/canonical Wnt signalling and healing of ischemic myocardium. Basic Res Cardiol 111:67
Barandon L, Couffinhal T, Ezan J et al (2003) Reduction of infarct size and prevention of cardiac rupture in transgenic mice overexpressing FrzA. Circulation 108:2282–2289
Chen L, Wu Q, Guo F, Xia B, Zuo J (2004) Expression of Dishevelled-1 in wound healing after acute myocardial infarction: possible involvement in myofibroblast proliferation and migration. J Cell Mol Med 8:257–264
Daskalopoulos EP, Dufeys C, Bertrand L, Beauloye C, Horman S (2016) AMPK in cardiac fibrosis and repair: actions beyond metabolic regulation. J Mol Cell Cardiol 91:188–200
Chang W, Zhang M, Meng Z, Yu Y, Yao F, Hatch GM, Chen L (2015) Berberine treatment prevents cardiac dysfunction and remodeling through activation of 5′-adenosine monophosphate-activated protein kinase in type 2 diabetic rats and in palmitate-induced hypertrophic H9c2 cells. Eur J Pharmacol 769:55–63
Li G, Xing W, Zhang M, Geng F, Yang H, Zhang H, Zhang X, Li J, Dong L, Gao F (2018) Antifibrotic cardioprotection of berberine via downregulating myocardial IGF-1 receptor-regulated MMP-2/MMP-9 expression in diabetic rats. Am J Physiol Heart Circ Physiol 315:H802–h813
Hori Y, Kashimoto T, Yonezawa T, Sano N, Saitoh R, Igarashi S, Chikazawa S, Kanai K, Hoshi F, Itoh N, Higuchi SI (2012) Matrix metalloproteinase-2 stimulates collagen-I expression through phosphorylation of focal adhesion kinase in rat cardiac fibroblasts. Am J Physiol Cell Physiol 303:C947–C953
Lindsey ML, Iyer RP, Zamilpa R, Yabluchanskiy A, DeLeon-Pennell K, Hall ME, Kaplan A, Zouein FA, Bratton D, Flynn ER, Cannon PL, Tian Y, Jin YF, Lange RA, Tokmina-Roszyk D, Fields GB, de Castro Brás LE (2015) A novel collagen matricryptin reduces left ventricular dilation post-myocardial infarction by promoting scar formation and angiogenesis. J Am Coll Cardiol 66:1364–1374
Lu K, Shen Y, He J, Liu G, Song W (2016) Berberine inhibits cardiac fibrosis of diabetic rats. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 32:1352–1355
Che Y, Shen DF, Wang ZP et al (2019) Protective role of berberine in isoprenaline-induced cardiac fibrosis in rats. BMC Cardiovasc Disord 19:219
Allijn IE, Czarny BM, Wang X et al (2017) Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J Control Release 247:127–133
Zhang T, Yang S, Du J (2014) Protective effects of berberine on isoproterenol-induced acute myocardial ischemia in rats through regulating HMGB1-TLR4 axis. Evid Based Complement Alternat Med 2014:1–8
Hong J, Yun CO (2019) Relaxin gene therapy: a promising new treatment option for various diseases with aberrant fibrosis or irregular angiogenesis. Mol Cell Endocrinol 487:80–84
Du X-J, Samuel CS, Gao X-M, Zhao L, Parry LJ, Tregear GW (2003) Increased myocardial collagen and ventricular diastolic dysfunction in relaxin deficient mice: a gender-specific phenotype. Cardiovasc Res 57:395–404
Samuel CS, Unemori EN, Mookerjee I et al (2004) Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 145:4125–4133
Moore X-l, Tan S-l, Lo C-y et al (2007) Relaxin antagonizes hypertrophy and apoptosis in neonatal rat cardiomyocytes. Endocrinology 148:1582–1589
Sasser JM, Molnar M, Baylis C (2011) Relaxin ameliorates hypertension and increases nitric oxide metabolite excretion in angiotensin II but not N ω-nitro-l-arginine methyl ester hypertensive rats. Hypertension 58:197–204
Zhang J, Qi Y-F, Geng B, Pan CS, Zhao J, Chen L, Yang J, Chang JK, Tang CS (2005) Effect of relaxin on myocardial ischemia injury induced by isoproterenol. Peptides 26:1632–1639
Dschietzig T, Richter C, Bartsch C et al (2001) The pregnancy hormone relaxin is a player in human heart failure. FASEB J 15:2187–2195
Xu Q, Lekgabe ED, Gao X-M et al (2008) Endogenous relaxin does not affect chronic pressure overload-induced cardiac hypertrophy and fibrosis. Endocrinology 149:476–482
Liao Y, Chen K, Dong X, Li W, Li G, Huang G, Song W, Chen L, Fang Y (2018) Berberine inhibits cardiac remodeling of heart failure after myocardial infarction by reducing myocardial cell apoptosis in rats. Exp Ther Med 16:2499–2505
Zhang YJ, Yang SH, Li MH, Iqbal J, Bourantas CV, Mi QY, Yu YH, Li JJ, Zhao SL, Tian NL, Chen SL (2014) Berberine attenuates adverse left ventricular remodeling and cardiac dysfunction after acute myocardial infarction in rats: role of autophagy. Clin Exp Pharmacol Physiol 41:995–1002
Ai F, Chen M, Yu B, Yang Y, Xu G, Gui F, Liu Z, Bai X, Chen Z (2015) Berberine regulates proliferation, collagen synthesis and cytokine secretion of cardiac fibroblasts via AMPK-mTOR-p70S6K signaling pathway. Int J Clin Exp Pathol 8:12509–12516
Zhao L, Sun L-N, Nie H-B, Wang X-L, Guan G-J (2014) Berberine improves kidney function in diabetic mice via AMPK activation. PLoS One 9:e113398–e113398
Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, Oh GT, Park HS, Lee KU, Lane MD, Kim JB (2009) Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. American Journal of Physiology-Endocrinology and Metabolism 296:E812–E819
Shen N, Huan Y, Shen Z-f (2012) Berberine inhibits mouse insulin gene promoter through activation of AMP activated protein kinase and may exert beneficial effect on pancreatic β-cell. Eur J Pharmacol 694:120–126
Boluyt MO, Li ZB, Loyd AM, Scalia AF, Cirrincione GM, Jackson RR (2004) The mTOR/p70 S6K signal transduction pathway plays a role in cardiac hypertrophy and influences expression of myosin heavy chain genes in vivo. Cardiovasc Drugs Ther 18:257–267
Liu X, Zhang X, Ye L, Yuan H (2016) Protective mechanisms of berberine against experimental autoimmune myocarditis in a rat model. Biomed Pharmacother 79:222–230
Lauer B, Padberg K, Schultheiss H-P, Strauer B-E (1994) Autoantibodies in against human ventricular myosin in sera of patients with acute and chronic myocarditis. J Am Coll Cardiol 23:146–153
Tajiri K, Imanaka-Yoshida K, Matsubara A, Tsujimura Y, Hiroe M, Naka T, Shimojo N, Sakai S, Aonuma K, Yasutomi Y (2012) Suppressor of cytokine signaling 1 DNA administration inhibits inflammatory and pathogenic responses in autoimmune myocarditis. J Immunol 189:2043–2053
Yu L, Li Q, Yu B et al (2016) Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxidative Med Cell Longev 2016
Qi M-y, Feng Y, Dai D-z, Li N, Cheng Y-s, Dai Y (2010) CPU86017, a berberine derivative, attenuates cardiac failure through normalizing calcium leakage and downregulated phospholamban and exerting antioxidant activity. Acta Pharmacol Sin 31:165–174
Leu T-H, Maa M-C (2002) The molecular mechanisms for the antitumorigenic effect of curcumin. Current Medicinal Chemistry-Anti-Cancer Agents 2:357–370
Jiménez-Osorio AS, Monroy A, Alavez S (2016) Curcumin and insulin resistance—molecular targets and clinical evidences. Biofactors 42:561–580
Hewlings SJ, Kalman DS (2017) Curcumin: a review of its’ effects on human health. Foods 6:92
Hazarey VK, Sakrikar AR, Ganvir SM (2015) Efficacy of curcumin in the treatment for oral submucous fibrosis-a randomized clinical trial. Journal of oral and maxillofacial pathology: JOMFP 19:145–152
Chen Y-N, Hsu S-L, Liao M-Y et al (2017) Ameliorative effect of curcumin-encapsulated hyaluronic acid–PLA nanoparticles on thioacetamide-induced murine hepatic fibrosis. Int J Environ Res Public Health 14:11
Tyagi N, Dash D, Singh R (2016) Curcumin inhibits paraquat induced lung inflammation and fibrosis by extracellular matrix modifications in mouse model. Inflammopharmacology 24:335–345
Hu Y, Mou L, Yang F, Tu H, Lin W (2016) Curcumin attenuates cyclosporine A-induced renal fibrosis by inhibiting hypermethylation of the klotho promoter. Mol Med Rep 14:3229–3236
Panahi Y, Khalili N, Sahebi E et al (2017) Curcuminoids modify lipid profile in type 2 diabetes mellitus: a randomized controlled trial. Complementary therapies in medicine 33:1–5
Yu W, Wu J, Cai F, Xiang J, Zha W, Fan D, Guo S, Ming Z, Liu C (2012) Curcumin alleviates diabetic cardiomyopathy in experimental diabetic rats. PLoS One 7:e52013
Wang NP, Wang ZF, Tootle S, Philip T, Zhao ZQ (2012) Curcumin promotes cardiac repair and ameliorates cardiac dysfunction following myocardial infarction. Br J Pharmacol 167:1550–1562
Meng Z, Yu X-h, Chen J, Li L, Li S (2014) Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-γ activation. Acta Pharmacol Sin 35:1247–1256
Khan R, Sheppard R (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-β1 in cardiomyopathy, valvular disease and arrhythmia. Immunology 118:10–24
Fang G, Chen S, Huang Q, Chen L, Liao D (2018) Curcumin suppresses cardiac fibroblasts activities by regulating the proliferation and cell cycle via the inhibition of the p38 MAPK/ERK signaling pathway. Mol Med Rep 18:1433–1438
Kong P, Christia P, Frangogiannis NG (2014) The pathogenesis of cardiac fibrosis. Cell Mol Life Sci 71:549–574
Ivey MJ, Tallquist MD (2016) Defining the cardiac fibroblast. Circulation Journal: CJ-16-1003
Carling D (2004) The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem Sci 29:18–24
Guo S, Meng XW, Yang XS, Liu XF, Ou-Yang CH, Liu C (2018) Curcumin administration suppresses collagen synthesis in the hearts of rats with experimental diabetes. Acta Pharmacol Sin 39:195–204
Declèves A-E, Sharma K (2014) Novel targets of antifibrotic and anti-inflammatory treatment in CKD. Nat Rev Nephrol 10:257–267
Sato N, Takasaka N, Yoshida M et al (2016) Metformin attenuates lung fibrosis development via NOX4 suppression. Respir Res 17:107
Lau WL, Khazaeli M, Savoj J, Manekia K, Bangash M, Thakurta RG, Dang A, Vaziri ND, Singh B (2018) Dietary tetrahydrocurcumin reduces renal fibrosis and cardiac hypertrophy in 5/6 nephrectomized rats. Pharmacol Res Perspect 6:e00385
Modlinger PS, Wilcox CS, Aslam S (2004) Nitric oxide, oxidative stress, and progression of chronic renal failure. In: Seminars in nephrology. Elsevier, pp 354–365
Cachofeiro V, Goicochea M, De Vinuesa SG, Oubiña P, Lahera V, Luño J (2008) Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease: new strategies to prevent cardiovascular risk in chronic kidney disease. Kidney Int 74:S4–S9
Massy Z, Maziere C, Kamel S et al (2005) Impact of inflammation and oxidative stress on vascular calcifications in chronic kidney disease. Pediatr Nephrol 20:380–382
Song KI, Park JY, Lee S, Lee D, Jang HJ, Kim SN, Ko H, Kim HY, Lee JW, Hwang GS, Kang KS, Yamabe N (2015) Protective effect of tetrahydrocurcumin against cisplatin-induced renal damage: in vitro and in vivo studies. Planta Med 81:286–291
Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK (2003) Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Kidney Int 63:179–185
Aminzadeh MA, Nicholas SB, Norris KC, Vaziri ND (2013) Role of impaired Nrf2 activation in the pathogenesis of oxidative stress and inflammation in chronic tubulo-interstitial nephropathy. Nephrology Dialysis Transplantation 28:2038–2045
Kim HJ, Vaziri ND (2010) Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. American journal of physiology-renal physiology 298:F662–F671
Li K, Zhai M, Jiang L et al (2019) Tetrahydrocurcumin ameliorates diabetic cardiomyopathy by attenuating high glucose-induced oxidative stress and fibrosis via activating the SIRT1 pathway. Oxidative Med Cell Longev 2019:6746907
Seferović PM, Paulus WJ (2015) Clinical diabetic cardiomyopathy: a two-faced disease with restrictive and dilated phenotypes. Eur Heart J 36:1718–1727
Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P (2001) IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II–mediated oxidative stress. Diabetes 50:1414–1424
Sung MM, Hamza SM, Dyck JR (2015) Myocardial metabolism in diabetic cardiomyopathy: potential therapeutic targets. Antioxid Redox Signal 22:1606–1630
Faria A, Persaud SJ (2017) Cardiac oxidative stress in diabetes: mechanisms and therapeutic potential. Pharmacol Ther 172:50–62
Berthiaume JM, Kurdys JG, Muntean DM, Rosca MG (2019) Mitochondrial NAD+/NADH redox state and diabetic cardiomyopathy. Antioxid Redox Signal 30:375–398
Ma S, Feng J, Zhang R, Chen J, Han D, Li X, Yang B, Li X, Fan M, Li C, Tian Z, Wang Y, Cao F (2017) SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxidative Med Cell Longev 2017:1–15
Carafa V, Rotili D, Forgione M et al (2016) Sirtuin functions and modulation: from chemistry to the clinic. Clin Epigenetics 8:61
Zhang B, Zhai M, Li B et al (2018) Honokiol ameliorates myocardial ischemia/reperfusion injury in type 1 diabetic rats by reducing oxidative stress and apoptosis through activating the SIRT1-Nrf2 signaling pathway. Oxidative Med Cell Longev 2018
Hashemzaei M, Heravi RE, Rezaee R, Roohbakhsh A, Karimi G (2017) Regulation of autophagy by some natural products as a potential therapeutic strategy for cardiovascular disorders. Eur J Pharmacol 802:44–51
Liu R, Zhang HB, Yang J, Wang JR, Liu JX, Li CL (2018) Curcumin alleviates isoproterenol-induced cardiac hypertrophy and fibrosis through inhibition of autophagy and activation of mTOR. Eur Rev Med Pharmacol Sci 22:7500–7508
Han J, Pan X-Y, Xu Y, Xiao Y, An Y, Tie L, Pan Y, Li XJ (2012) Curcumin induces autophagy to protect vascular endothelial cell survival from oxidative stress damage. Autophagy 8:812–825
Yang K, Xu C, Li X, Jiang H (2013) Combination of D942 with curcumin protects cardiomyocytes from ischemic damage through promoting autophagy. J Cardiovasc Pharmacol Ther 18:570–581
Jia Y, Yue Y, Hu D-N, Chen J-L, Zhou J-B (2017) Human aqueous humor levels of transforming growth factor-β2: association with matrix metalloproteinases/tissue inhibitors of matrix metalloproteinases. Biomedical reports 7:573–578
Gao H, Frost MR, Siegwart JT Jr, Norton TT (2011) Patterns of mRNA and protein expression during minus-lens compensation and recovery in tree shrew sclera. Mol Vis 17:903
McBrien NA, Gentle A (2003) Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res 22:307–338
Shelton L, Rada JS (2007) Effects of cyclic mechanical stretch on extracellular matrix synthesis by human scleral fibroblasts. Exp Eye Res 84:314–322
Yao Q-H, Wang D-Q, Cui C-C et al (2004) Curcumin ameliorates left ventricular function in rabbits with pressure overload: inhibition of the remodeling of the left ventricular collagen network associated with suppression of myocardial tumor necrosis factor-α and matrix metalloproteinase-2 expression. Biol Pharm Bull 27:198–202
Ma J, Ma SY, Ding CH (2017) Curcumin reduces cardiac fibrosis by inhibiting myofibroblast differentiation and decreasing transforming growth factor beta1 and matrix metalloproteinase 9/tissue inhibitor of metalloproteinase 1. Chin J Integr Med 23:362–369
Shimosawa T (2013) Salt, the renin–angiotensin–aldosterone system and resistant hypertension. Hypertens Res 36:657–660
Meng Z, Yu XH, Chen J, Li L, Li S (2014) Curcumin attenuates cardiac fibrosis in spontaneously hypertensive rats through PPAR-gamma activation. Acta Pharmacol Sin 35:1247–1256
Askari AT, Shishehbor MH, Kaminski MA, Riley MJ, Hsu A, Lincoff AM, GUSTO-V Investigators (2009) The association between early ventricular arrhythmias, renin-angiotensin-aldosterone system antagonism, and mortality in patients with ST-segment-elevation myocardial infarction: insights from global use of strategies to open coronary arteries (GUSTO) V. Am Heart J 158:238–243
de Cavanagh EM, Ferder M, Inserra F, Ferder L (2009) Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Phys Heart Circ Phys 296:H550–H558
Siddesha JM, Valente AJ, Sakamuri SS, Yoshida T, Gardner JD, Somanna N, Takahashi C, Noda M, Chandrasekar B (2013) Angiotensin II stimulates cardiac fibroblast migration via the differential regulation of matrixins and RECK. J Mol Cell Cardiol 65:9–18
Ghosh AK, Vaughan DE (2012) PAI-1 in tissue fibrosis. J Cell Physiol 227:493–507
Hao GH, Niu XL, Gao DF, Wei J, Wang NP (2008) Agonists at PPAR-γ suppress angiotensin II-induced production of plasminogen activator inhibitor-1 and extracellular matrix in rat cardiac fibroblasts. Br J Pharmacol 153:1409–1419
Weisberg AD, Albornoz F, Griffin JP, Crandall DL, Elokdah H, Fogo AB, Vaughan DE, Brown NJ (2005) Pharmacological inhibition and genetic deficiency of plasminogen activator inhibitor-1 attenuates angiotensin II/salt-induced aortic remodeling. Arterioscler Thromb Vasc Biol 25:365–371
Liu X, Gai Y, Liu F, Gao W, Zhang Y, Xu M, Li Z (2010) Trimetazidine inhibits pressure overload-induced cardiac fibrosis through NADPH oxidase–ROS–CTGF pathway. Cardiovasc Res 88:150–158
Gao D-F, Niu X-L, Hao G-H, Peng N, Wei J, Ning N, Wang NP (2007) Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells––role of PPAR-γ in vascular fibrosis. Biochem Pharmacol 73:185–197
Ahmed MS, Øie E, Vinge LE et al (2004) Connective tissue growth factor—a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol 36:393–404
Song K, Peng S, Sun Z, Li H, Yang R (2011) Curcumin suppresses TGF-β signaling by inhibition of TGIF degradation in scleroderma fibroblasts. Biochem Biophys Res Commun 411:821–825
Hu Y, Liang H, Du Y, Zhu Y, Wang X (2010) Curcumin inhibits transforming growth factor-β activity via inhibition of Smad signaling in HK-2 cells. Am J Nephrol 31:332–341
Ryu HW, Kim SP, Lee KS, Cho JW (2012) Curcumin induced decreased expression of type I collagen in human skin fibroblast through down-regulation of Smad2/3 expressions. Korean J Dermatol 50:1
Dobaczewski M, Chen W, Frangogiannis NG (2011) Transforming growth factor (TGF)-β signaling in cardiac remodeling. J Mol Cell Cardiol 51:600–606
Ji Y, Liu J, Wang Z, Liu N, Gou W (2009) PPAR γ agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Investig 89:887–902
Derosa G, Maffioli P (2012) Peroxisome proliferator-activated receptor-γ (PPAR-γ) agonists on glycemic control, lipid profile and cardiovascular risk. Curr Mol Pharmacol 5:272–281
Goyal S, Arora S, Bhatt TK, Das P, Sharma A, Kumari S, Arya DS (2010) Modulation of PPAR-γ by telmisartan protects the heart against myocardial infarction in experimental diabetes. Chem Biol Interact 185:271–280
Oemar BS, Werner A, Garnier J-M, Do DD, Godoy N, Nauck M, Ma¨rz W, Rupp J, Pech M, Lu¨scher TF (1997) Human connective tissue growth factor is expressed in advanced atherosclerotic lesions. Circulation 95:831–839
Hsu C-P, Zhai P, Yamamoto T, Maejima Y, Matsushima S, Hariharan N, Shao D, Takagi H, Oka S, Sadoshima J (2010) Silent information regulator 1 protects the heart from ischemia/reperfusion. Circulation 122:2170–2182
Wu Y, Liu X, Zhou Q et al (2015) Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. Toxicol Appl Pharmacol 289:163–176
Rizk SM, El-Maraghy SA, Nassar NN (2014) A novel role for SIRT-1 in L-arginine protection against STZ induced myocardial fibrosis in rats. PloS one 9
Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D, Jin Z (2013) SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med 65:667–679
Xiao J, Sheng X, Zhang X, Guo M, Ji X (2016) Curcumin protects against myocardial infarction-induced cardiac fibrosis via SIRT1 activation in vivo and in vitro. Drug Des Devel Ther 10:1267–1277
Najafpour Boushehri S, Karimbeiki R, Ghasempour S, Ghalishourani SS, Pourmasoumi M, Hadi A, Mbabazi M, pour ZK, Assarroudi M, Mahmoodi M, Khosravi A, Mansour-Ghanaei F, Joukar F (2020) The efficacy of sour tea (Hibiscus sabdariffa L.) on selected cardiovascular disease risk factors: a systematic review and meta-analysis of randomized clinical trials. Phytother Res 34:329–339
Nguyen QD, Pham TN, Binh MLT, et al. (2020) Effects of extraction conditions on antioxidant activities of Roselle (Hibiscus sabdariffa L.) extracts. In: Materials Science Forum. Trans Tech Publ, pp 201-206
Huang T-W, Chang C-L, Kao E-S, Lin J-H (2015) Effect of Hibiscus sabdariffa extract on high fat diet–induced obesity and liver damage in hamsters. Food Nutr Res 59:29018
Mohammed Yusof NL, Zainalabidin S, Mohd Fauzi N, Budin SB (2018) Hibiscus sabdariffa (roselle) polyphenol-rich extract averts cardiac functional and structural abnormalities in type 1 diabetic rats. Appl Physiol Nutr Metab 43:1224–1232
Miki T, Yuda S, Kouzu H, Miura T (2013) Diabetic cardiomyopathy: pathophysiology and clinical features. Heart Fail Rev 18:149–166
Vanessa Fiorentino T, Prioletta A, Zuo P, Folli F (2013) Hyperglycemia-induced oxidative stress and its role in diabetes mellitus related cardiovascular diseases. Curr Pharm Des 19:5695–5703
Fuentes-Antrás J, Picatoste B, Ramírez E, Egido J, Tuñón J, Lorenzo Ó (2015) Targeting metabolic disturbance in the diabetic heart. Cardiovasc Diabetol 14:17
Si LY-N, Ali SAM, Latip J, Fauzi NM, Budin SB, Zainalabidin S (2017) Roselle is cardioprotective in diet-induced obesity rat model with myocardial infarction. Life Sci 191:157–165
Ali SS, Mohamed SFA, Rozalei NH, Boon YW, Zainalabidin S (2019) Anti-fibrotic actions of Roselle extract in rat model of myocardial infarction. Cardiovasc Toxicol 19:72–81
Goel A, Pothineni NV, Singhal M, Paydak H, Saldeen T, Mehta JL (2018) Fish, fish oils and cardioprotection: promise or fish tale? International journal of molecular sciences 19
Parikh M, Raj P, Austria JA, Yu L, Garg B, Netticadan T, Pierce GN (2019) Dietary flaxseed protects against ventricular arrhythmias and left ventricular dilation after a myocardial infarction. J Nutr Biochem 71:63–71
Parikh M, Netticadan T, Pierce GN (2018) Flaxseed: its bioactive components and their cardiovascular benefits. Am J Phys Heart Circ Phys 314:H146–H159
Rodriguez-Leyva D, Weighell W, Edel AL, LaVallee R, Dibrov E, Pinneker R, Maddaford TG, Ramjiawan B, Aliani M, Guzman R, Pierce GN (2013) Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension 62:1081–1089
Francis AA, Deniset JF, Austria JA, LaValleé RK, Maddaford GG, Hedley TE, Dibrov E, Pierce GN (2013) Effects of dietary flaxseed on atherosclerotic plaque regression. Am J Phys Heart Circ Phys 304:H1743–H1751
Bassett CM, McCullough RS, Edel AL, Patenaude A, LaVallee RK, Pierce GN (2011) The α-linolenic acid content of flaxseed can prevent the atherogenic effects of dietary trans fat. Am J Phys Heart Circ Phys 301:H2220–H2226
Ander BP, Weber AR, Rampersad PP, Gilchrist JS, Pierce GN, Lukas A (2004) Dietary flaxseed protects against ventricular fibrillation induced by ischemia-reperfusion in normal and hypercholesterolemic rabbits. J Nutr 134:3250–3256
Ganguly R, Hasanally D, Stamenkovic A, Maddaford TG, Chaudhary R, Pierce GN, Ravandi A (2018) Alpha linolenic acid decreases apoptosis and oxidized phospholipids in cardiomyocytes during ischemia/reperfusion. Mol Cell Biochem 437:163–175
Pfeffer MA, Braunwald E, Moyé LA, Basta L, Brown EJ Jr, Cuddy TE, Davis BR, Geltman EM, Goldman S, Flaker GC, Klein M, Lamas GA, Packer M, Rouleau J, Rouleau JL, Rutherford J, Wertheimer JH, Hawkins CM (1992) Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction: results of the Survival and Ventricular Enlargement Trial. N Engl J Med 327:669–677
St John Sutton M, Lee D, Rouleau JL, Goldman S, Plappert T, Braunwald E, Pfeffer MA (2003) Left ventricular remodeling and ventricular arrhythmias after myocardial infarction. Circulation 107:2577–2582
Seropian IM, Toldo S, Van Tassell BW, Abbate A (2014) Anti-inflammatory strategies for ventricular remodeling following ST-segment elevation acute myocardial infarction. J Am Coll Cardiol 63:1593–1603
Yamazaki T, Izumi Y, Nakamura Y, Yamashita N, Fujiki H, Osada-Oka M, Shiota M, Hanatani A, Shimada K, Iwao H, Yoshiyama M (2012) Tolvaptan improves left ventricular dysfunction after myocardial infarction in rats. Circ Heart Fail 5:794–802
Dupasquier CM, Dibrov E, Kneesh AL, Cheung PK, Lee KG, Alexander HK, Yeganeh BK, Moghadasian MH, Pierce GN (2007) Dietary flaxseed inhibits atherosclerosis in the LDL receptor-deficient mouse in part through antiproliferative and anti-inflammatory actions. Am J Phys Heart Circ Phys 293:H2394–H2402
Voloshenyuk TG, Hart AD, Khoutorova E, Gardner JD (2011) TNF-α increases cardiac fibroblast lysyl oxidase expression through TGF-β and PI3Kinase signaling pathways. Biochem Biophys Res Commun 413:370–375
Kania G, Blyszczuk P, Eriksson U (2009) Mechanisms of cardiac fibrosis in inflammatory heart disease. Trends in cardiovascular medicine 19:247–252
Covas M-I (2007) Olive oil and the cardiovascular system. Pharmacol Res 55:175–186
Huang CL, Sumpio BE (2008) Olive oil, the mediterranean diet, and cardiovascular health. J Am Coll Surg 207:407–416
Martinez-Gonzalez MA, Bes-Rastrollo M, Serra-Majem L, Lairon D, Estruch R, Trichopoulou A (2009) Mediterranean food pattern and the primary prevention of chronic disease: recent developments. Nutr Rev 67:S111–S116
Manna C, Migliardi V, Golino P, Scognamiglio A, Galletti P, Chiariello M, Zappia V (2004) Oleuropein prevents oxidative myocardial injury induced by ischemia and reperfusion. J Nutr Biochem 15:461–466
Miles EA, Zoubouli P, Calder PC (2005) Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 21:389–394
Poudyal H, Campbell F, Brown L (2010) Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate–, high fat–fed rats. J Nutr 140:946–953
Chung LY (2006) The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin, and allyl disulfide. J Med Food 9:205–213
Rabinkov A, Miron T, Konstantinovski L, Wilchek M, Mirelman D, Weiner L (1998) The mode of action of allicin: trapping of radicals and interaction with thiol containing proteins. Biochimica et Biophysica Acta (BBA)-General Subjects 1379: 233-244
Hasan N, Yusuf N, Toossi Z, Islam N (2006) Suppression of Mycobacterium tuberculosis induced reactive oxygen species (ROS) and TNF-α mRNA expression in human monocytes by allicin. FEBS Lett 580:2517–2522
Mirelman D, Monheit D, Varon S (1987) Inhibition of growth of Entamoeba histolytica by allicin, the active principle of garlic extract (Allium sativum). J Infect Dis 156:243–244
Liu C, Cao F, Tang Q-Z, Yan L, Dong YG, Zhu LH, Wang L, Bian ZY, Li H (2010) Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J Nutr Biochem 21:1238–1250
Prasad K, Laxdal VA, Yu M, Raney BL (1995) Antioxidant activity of allicin, an active principle in garlic. Mol Cell Biochem 148:183–189
Horev-Azaria L, Eliav S, Izigov N, Pri-Chen S, Mirelman D, Miron T, Rabinkov A, Wilchek M, Jacob-Hirsch J, Amariglio N, Savion N (2009) Allicin up-regulates cellular glutathione level in vascular endothelial cells. Eur J Nutr 48:67–74
Schwartz IF, Hershkovitz R, Iaina A et al (2002) Garlic attenuates nitric oxide Production in rat cardiac myocytes through inhibition of inducible nitric oxide synthase and the arginine transporter CAT-2 (cationic amino acid transporter-2). Clin Sci 102:487–493
Sun X, Ku DD (2006) Allicin in garlic protects against coronary endothelial dysfunction and right heart hypertrophy in pulmonary hypertensive rats. Am J Phys Heart Circ Phys 291:H2431–H2438
Tsujimoto I, Hikoso S, Yamaguchi O, Kashiwase K, Nakai A, Takeda T, Watanabe T, Taniike M, Matsumura Y, Nishida K, Hori M, Kogo M, Otsu K (2005) The antioxidant edaravone attenuates pressure overload–induced left ventricular hypertrophy. Hypertension 45:921–926
Li H-L, Huang Y, Zhang C-N, Liu G, Wei YS, Wang AB, Liu YQ, Hui RT, Wei C, Williams GM, Liu DP, Liang CC (2006) Epigallocathechin-3 gallate inhibits cardiac hypertrophy through blocking reactive oxidative species-dependent and-independent signal pathways. Free Radic Biol Med 40:1756–1775
Kwon SH, Pimentel DR, Remondino A, Sawyer DB, Colucci WS (2003) H2O2 regulates cardiac myocyte phenotype via concentration-dependent activation of distinct kinase pathways. J Mol Cell Cardiol 35:615–621
Zhan C-Y, Tang J-H, Zhou D-X, Li Z-H (2014) Effects of tanshinone IIA on the transforming growth factor β1/Smad signaling pathway in rat cardiac fibroblasts. Indian journal of pharmacology 46:633
Tang B, Zhu B, Liang Y, Bi L, Hu Z, Chen B, Zhang K, Zhu J (2011) Asiaticoside suppresses collagen expression and TGF-β/Smad signaling through inducing Smad7 and inhibiting TGF-βRI and TGF-βRII in keloid fibroblasts. Arch Dermatol Res 303:563–572
Leask A (2007) TGFβ, cardiac fibroblasts, and the fibrotic response. Cardiovasc Res 74:207–212
Yoshimatsu Y, Watabe T (2011) Roles of TGF-β signals in endothelial-mesenchymal transition during cardiac fibrosis. Int J Inflamm 2011
Li SC, Ma LN, Chen J, Li YK (2016) Effect of allicin on myocardial fibrosis after myocardial infarction in rats and its relationship with TGFbeta/Smads signal transduction. Zhongguo Zhong Yao Za Zhi 41:2517–2521
Nakano Y, Matoba T, Tokutome M, Funamoto D, Katsuki S, Ikeda G, Nagaoka K, Ishikita A, Nakano K, Koga JI, Sunagawa K, Egashira K (2016) Nanoparticle-mediated delivery of irbesartan induces cardioprotection from myocardial ischemia-reperfusion injury by antagonizing monocyte-mediated inflammation. Sci Rep 6:29601
Yue T-L, Bao W, Gu J-L et al (2005) Rosiglitazone treatment in Zucker diabetic fatty rats is associated with ameliorated cardiac insulin resistance and protection from ischemia/reperfusion-induced myocardial injury. Diabetes 54:554–562
Vasheghani F, Zhang Y, Li Y-H, Blati M, Fahmi H, Lussier B, Roughley P, Lagares D, Endisha H, Saffar B, Lajeunesse D, Marshall WK, Rampersaud YR, Mahomed NN, Gandhi R, Pelletier JP, Martel-Pelletier J, Kapoor M (2015) PPARγ deficiency results in severe, accelerated osteoarthritis associated with aberrant mTOR signalling in the articular cartilage. Ann Rheum Dis 74:569–578
Torigoe Y, Takahashi N, Hara M, Yoshimatsu H, Saikawa T (2009) Adrenomedullin improves cardiac expression of heat-shock protein 72 and tolerance against ischemia/reperfusion injury in insulin-resistant rats. Endocrinology 150:1450–1455
Kim Y-J, Park K-J, Song J-K et al (2012) The PPARγ agonist protects cardiomyocytes from oxidative stress and apoptosis via thioredoxin overexpression. Biosci Biotechnol Biochem 76:2181–2187
Yue LJ, Zhu XY, Li RS, Chang HJ, Gong B, Tian CC, Liu C, Xue YX, Zhou Q, Xu TS, Wang DJ (2019) Sallylcysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy. Int J Mol Med 44:1943–1951
Takimoto E, Kass DA (2007) Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 49:241–248
Schwinger RH, Bundgaard H, Müller-Ehmsen J, Kjeldsen K (2003) The Na, K-ATPase in the failing human heart. Cardiovasc Res 57:913–920
Khatua TN, Borkar RM, Mohammed SA, Dinda AK, Srinivas R, Banerjee SK (2017) Novel sulfur metabolites of garlic attenuate cardiac hypertrophy and remodeling through induction of Na+/K+-ATPase expression. Front Pharmacol 8:18
Chen T, Li J, Liu J, Li N, Wang S, Liu H, Zeng M, Zhang Y, Bu P (2015) Activation of SIRT3 by resveratrol ameliorates cardiac fibrosis and improves cardiac function via the TGF-β/Smad3 pathway. Am J Phys Heart Circ Phys 308:H424–H434
Venkatachalam K, Mummidi S, Cortez DM, Prabhu SD, Valente AJ, Chandrasekar B (2008) Resveratrol inhibits high glucose-induced PI3K/Akt/ERK-dependent interleukin-17 expression in primary mouse cardiac fibroblasts. Am J Phys Heart Circ Phys 294:H2078–H2087
Wu H, Li G-N, Xie J et al (2016) Resveratrol ameliorates myocardial fibrosis by inhibiting ROS/ERK/TGF-β/periostin pathway in STZ-induced diabetic mice. BMC Cardiovasc Disord 16:5
Zou LX, Chen C, Yan X, et al. (2019) Resveratrol attenuates pressure overload-induced cardiac fibrosis and diastolic dysfunction via PTEN/AKT/Smad2/3 and NF-κB signaling pathways. Molecular nutrition & food research: 1900418
Author information
Authors and Affiliations
Contributions
JH and ZA contributed in conception, design, and drafting of the manuscript. LM, ZR, and PZ contributed in data collection and manuscript drafting. All authors approved the final version for submission. JH oversaw the study.
Corresponding authors
Ethics declarations
Competing interests
ŽR has received honoraria from SanofiAventis. The rest of the authors declare no conflict of interest.
Ethics approval and consent to participate
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zivarpour, P., Reiner, Ž., Hallajzadeh, J. et al. The effect of nutraceuticals on multiple signaling pathways in cardiac fibrosis injury and repair. Heart Fail Rev 27, 321–336 (2022). https://doi.org/10.1007/s10741-020-09980-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09980-6