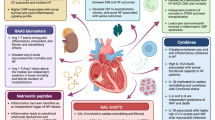
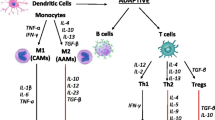
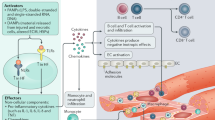
Abstract
Inflammation has long been known to play a role in heart failure (HF). Earlier studies demonstrated that inflammation contributes to the pathogenesis of HF with reduced ejection fraction (HFrEF), and the knowledge about molecules and cell types specifically involved in inflammatory events has been constantly increased ever since. However, conflicting results of several trials with anti-inflammatory treatments led to the conclusions that inflammation does participate in the progression of HFrEF, but more likely it is not the primary event. Conversely, it has been suggested that inflammation drives the development of HF with preserved ejection fraction (HFpEF). Recently the pharmacological blockade of interleukin-1 has been shown to prevent HF hospitalization and mortality in patients with prior myocardial infarction, lending renewed support to the hypothesis that inflammation is a promising therapeutic target in HF. Inflammation has also been proposed to underlie both HF and commonly associated conditions, such as chronic kidney disease or cancer. Within this last paradigm, an emergent role has been ascribed to clonal hematopoiesis of indeterminate potential. Here, we summarize the recent evidence about the role of inflammation in HF, highlighting the similarities and differences in HFrEF vs. HFpEF, and discuss the diagnostic and therapeutic opportunities raised by antinflammatory-based approaches.


Similar content being viewed by others
References
Dickstein K, Cohen-Solal A, Filippatos G, McMurray JJV, Ponikowski P, Poole-Wilson PA, Strömberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Vahanian A, Camm J, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Document R, Tendera M, Auricchio A, Bax J, Böhm M, Corrà U, della Bella P, Elliott PM, Follath F, Gheorghiade M, Hasin Y, Hernborg A, Jaarsma T, Komajda M, Kornowski R, Piepoli M, Prendergast B, Tavazzi L, Vachiery J-L, Verheugt FWA, Zamorano JL, Zannad F, M. Authors/Task Force, E.S.C.C.f.P. Guidelines (2008) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the heart failure association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 29:2388–2442
P. Ponikowski, A.A. Voors, S.D. Anker, H. Bueno, J.G.F. Cleland, A.J.S. Coats, V. Falk, J.R. González-Juanatey, V.-P. Harjola, E.A. Jankowska, M. Jessup, C. Linde, P. Nihoyannopoulos, J.T. Parissis, B. Pieske, J.P. Riley, G.M.C. Rosano, L.M. Ruilope, F. Ruschitzka, F.H. Rutten, P. van der Meer, G. Filippatos, J.J.V. McMurray, V. Aboyans, S. Achenbach, S. Agewall, N. Al-Attar, J.J. Atherton, J. Bauersachs, A. John Camm, S. Carerj, C. Ceconi, A. Coca, P. Elliott, Ç. Erol, J. Ezekowitz, C. Fernández-Golfín, D. Fitzsimons, M. Guazzi, M. Guenoun, G. Hasenfuss, G. Hindricks, A.W. Hoes, B. Iung, T. Jaarsma, P. Kirchhof, J. Knuuti, P. Kolh, S. Konstantinides, M. Lainscak, P. Lancellotti, G.Y.H. Lip, F. Maisano, C. Mueller, M.C. Petrie, M.F. Piepoli, S.G. Priori, A. Torbicki, H. Tsutsui, D.J. van Veldhuisen, S. Windecker, C. Yancy, J.L. Zamorano, J.L. Zamorano, V. Aboyans, S. Achenbach, S. Agewall, L. Badimon, G. Barón-Esquivias, H. Baumgartner, J.J. Bax, H. Bueno, S. Carerj, V. Dean, Ç. Erol, D. Fitzsimons, O. Gaemperli, P. Kirchhof, P. Kolh, P. Lancellotti, G.Y.H. Lip, P. Nihoyannopoulos, M.F. Piepoli, P. Ponikowski, M. Roffi, A. Torbicki, A. Vaz Carneiro, S. Windecker, H.S. Sisakian, E. Isayev, A. Kurlianskaya, W. Mullens, M. Tokmakova, P. Agathangelou, V. Melenovsky, H. Wiggers, M. Hassanein, T. Uuetoa, J. Lommi, E.S. Kostovska, Y. Juillière, A. Aladashvili, A. Luchner, C. Chrysohoou, N. Nyolczas, G. Thorgeirsson, J. Marc Weinstein, A. Di Lenarda, N. Aidargaliyeva, G. Bajraktari, M. Beishenkulov, G. Kamzola, T. Abdel-Massih, J. Čelutkienė, S. Noppe, A. Cassar, E. Vataman, S. Abir-Khalil, P. van Pol, R. Mo, E. Straburzyńska-Migaj, C. Fonseca, O. Chioncel, E. Shlyakhto, P. Otasevic, E. Goncalvesová, M. Lainscak, B. Díaz Molina, M. Schaufelberger, T. Suter, M.B. Yılmaz, L. Voronkov, C. Davies, 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure, Eur Heart J (2016)
Yancy Clyde W, Jessup M, Bozkurt B, Butler J, Casey Donald E, Drazner Mark H, Fonarow Gregg C, Geraci Stephen A, Horwich T, Januzzi James L, Johnson Maryl R, Kasper Edward K, Levy Wayne C, Masoudi Frederick A, McBride Patrick E, McMurray John JV, Mitchell Judith E, Peterson Pamela N, Riegel B, Sam F, Stevenson Lynne W, Tang WHW, Tsai Emily J, Wilkoff Bruce L (2013) 2013 ACCF/AHA guideline for the management of heart failure. Circulation 128:e240–e327
González A, Ravassa S, Beaumont J, López B, Díez J (2011) New targets to treat the structural remodeling of the myocardium. J Am Coll Cardio 58:1833–1843
Brouwers FP, De Boer RA, Van Der Harst P, Voors AA, Gansevoort RT, Bakker SJ, Hillege HL, Van Veldhuisen DJ, Van Gilst WH (2013) Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur Heart J 34:1424–1431
Paulus WJ, Tschöpe C (2013) A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 62:263–271
Dunlay SM, Roger VL, Redfield MM (2017) Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol 14:591
Mann Douglas L (2015) Innate immunity and the failing heart: the cytokine hypothesis revisited. Circ Res 116(7):1254–1268
Ngkelo A, Richart A, Kirk JA, Bonnin P, Vilar J, Lemitre M, Marck P, Branchereau M, Le Gall S, Renault N, Guerin C, Ranek MJ, Kervadec A, Danelli L, Gautier G, Blank U, Launay P, Camerer E, Bruneval P, Menasche P, Heymes C, Luche E, Casteilla L, Cousin B, Rodewald H-R, Kass DA, Silvestre J-S (2016) Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J Exp Med 213:1353–1374
Thackeray JT, Hupe HC, Wang Y, Bankstahl JP, Berding G, Ross TL, Bauersachs J, Wollert KC, Bengel FM (2018) Myocardial inflammation predicts remodeling and neuroinflammation after myocardial infarction. J Am Coll Cardio 71(3):263–275
Borlaug BA, Olson TP, Lam CSP, Flood KS, Lerman A, Johnson BD, Redfield MM (2010) Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 56:845–854
Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, Carriero R, Termanini A, Colombo FS, Jachetti E, Panico C, Faggian G, Fumero A, Torracca L, Molgora M, Cibella J, Pagiatakis C, Brummelman J, Alvisi G, Mazza EMC, Colombo MP, Lugli E, Condorelli G, Kallikourdis M (2019) Single cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation
Czubryt MP (2012) Common threads in cardiac fibrosis, infarct scar formation, and wound healing. Fibrogenesis Tissue Repair 5:19
Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3:e442
Weirather J, Frantz S Role of the innate immune system in ischemic heart failure. In: Blankesteijn WM, Altara R (eds) Inflammation in heart failure, Chapter 22015, pp 19–38
Cao Dian J, Schiattarella Gabriele G, Villalobos E, Jiang N, May Herman I, Li T, Chen Zhijian J, Gillette Thomas G, Hill Joseph A (2018) Cytosolic DNA sensing promotes macrophage transformation and governs myocardial ischemic injury. Circulation 137:2613–2634
Jahng JWS, Song E, Sweeney G (2016) Crosstalk between the heart and peripheral organs in heart failure. Exp Mol Med 48:e217–e217
Deswal A, Petersen Nancy J, Feldman Arthur M, Young James B, White Bill G, Mann Douglas L (2001) Cytokines and cytokine receptors in advanced heart failure: an analysis of the cytokine database from the Vesnarinone trial (VEST). Circulation 103:2055–2059
Wu C-K, Lee J-K, Chiang F-T, Yang C-H, Huang S-W, Hwang J-J, Lin J-L, Tseng C-D, Chen J-J, Tsai C-T (2011) Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Cri Care Med 39:984–992
Krown KA, Page MT, Nguyen C, Zechner D, Gutierrez V, Comstock KL, Glembotski CC, Quintana PJ, Sabbadini RA (1996) Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J Clin Inv 98:2854–2865
Janicki JS, Brower GL, Gardner JD, Chancey AL, Stewart JA Jr (2004) The dynamic interaction between matrix metalloproteinase activity and adverse myocardial remodeling. Heart Fail Rev 9(1):33–42
Bers DM (2006) Altered cardiac myocyte Ca regulation in heart failure. Physiology 21:380–387
Bers D (2001) Excitation–contraction coupling and cardiac contractile force. Kluwer Academic, Dordrecht
Bers DM (2014) Cardiac sarcoplasmic reticulum calcium leak: basis and roles in cardiac dysfunction. Annu Rev Physiol 76:107–127
Tsai C-T, Wu C-K, Lee J-K, Chang S-N, Kuo Y-M, Wang Y-C, Lai L-P, Chiang F-T, Hwang J-J, Lin J-L (2015) TNF-α down-regulates sarcoplasmic reticulum Ca2+ ATPase expression and leads to left ventricular diastolic dysfunction through binding of NF-κB to promoter response element. Cardiovasc Res 105:318–329
Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P (1999) Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res 84(6):713–721
Kirchhefer U, Schmitz W, Scholz H, Neumann J (1999) Activity of cAMP-dependent protein kinase and Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human hearts. Cardiovasc Res 42(1):254–261
Feng N, Anderson ME (2017) CaMKII is a nodal signal for multiple programmed cell death pathways in heart. J Mol Cell Cardiol 103:102–109
Singh MV, Anderson ME (2011) Is CaMKII a link between inflammation and hypertrophy in heart? J Mol Med 89:537–543
Singh MV, Swaminathan PD, Luczak ED, Kutschke W, Weiss RM, Anderson ME (2012) MyD88 mediated inflammatory signaling leads to CaMKII oxidation, cardiac hypertrophy and death after myocardial infarction. J Mol Cell Cardio 52:1135–1144
Greenhaff PL (2001) The creatine-phosphocreatine system: there's more than one song in its repertoire. J Physiol 537:657
Mekhfi H, Veksler V, Mateo P, Maupoil V, Rochette L, Ventura-Clapier R (1996) Creatine kinase is the main target of reactive oxygen species in cardiac myofibrils. Circ Res 78:1016–1027
Khuchua ZA, Ventura-Clapier R, Kuznetsov AV, Grishin MN, Saks VA (1989) Alterations in the creatine kinase system in the myocardium of cardiomyopathic hamsters. Biochem Biophys Res Comm 165:748–757
Nascimben L, Friedrich J, Liao R, Pauletto P, Pessina AC, Ingwall JS (1995) Enalapril treatment increases cardiac performance and energy reserve via the creatine kinase reaction in myocardium of Syrian myopathic hamsters with advanced heart failure. Circulation 91:1824–1833
Neubauer S, Horn M, Naumann A, Tian R, Hu K, Laser M, Friedrich J, Gaudron P, Schnackerz K, Ingwall JS (1995) Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J Clin Invest 95:1092
Pinz I, Ostroy SE, Hoyer K, Osinska H, Robbins J, Molkentin JD, Ingwall JS (2008) Calcineurin-induced energy wasting in a transgenic mouse model of heart failure. Am J Phys 294:H1459–H1466
Szeto HH (2014) First-in-class cardiolipin-protective compound as a therapeutic agent to restore mitochondrial bioenergetics. Br J Pharmacol 171:2029–2050
Bertero E, Maack C (2018) Calcium signaling and reactive oxygen species in mitochondria. Circ Res 122:1460–1478
Neubauer S, Horn M, Pabst T, Gödde M, Lübke D, Jilling B, Hahn D, Ertl G (1995) Contributions of 31P-magnetic resonance spectroscopy to the understanding of dilated heart muscle disease. Eur Heart J 16:115–118
Hamdani N, Franssen C, Lourenço A, Falcão-Pires I, Fontoura D, Leite S, Plettig L, López B, Ottenheijm CA, Becher PM, González A, Tschöpe C, DÃez J, Linke WA, Leite-Moreira AF, Paulus WJ (2013) Myocardial titin hypophosphorylation importantly contributes to heart failure with preserved ejection fraction in a rat metabolic risk model. Circ Heart Fail 6:1239–1249
Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA (2019) Nitrosative stress drives heart failure with preserved ejection fraction. Nature 568:351–356
Tong D, Schiattarella GG, Jiang N, May HI, Lavandero S, Gillette TG, Hill JA (2019) Female sex is protective in a preclinical model of heart failure with preserved ejection fraction. Circulation 140(21):1769–1771
Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS (1997) Protection from obesity-induced insulin resistance in mice lacking TNF-α function. Nature 389:610
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Inv 112(12):1821–1830
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. J Am Coll Cardio 286:327–334
Sanders-van Wijk S, van Empel V, Davarzani N, Maeder MT, Handschin R, Pfisterer ME, Brunner-La Rocca HP (2015) Circulating biomarkers of distinct pathophysiological pathways in heart failure with preserved vs. reduced left ventricular ejection fraction. Eur J Heart Fail 17:1006–1014
Scherzer R, Shah Sanjiv J, Secemsky E, Butler J, Grunfeld C, Shlipak Michael G, Hsue Priscilla Y (2018) Association of biomarker clusters with cardiac phenotypes and mortality in patients with HIV infection. Circulation 11(4):e004312
Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss H-P, Tschöpe C (2011) Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circ Heart Fail 4:44–52
Franssen C, Chen S, Unger A, Korkmaz HI, De Keulenaer GW, Tschöpe C, Leite-Moreira AF, Musters R, Niessen HWM, Linke WA, Paulus WJ, Hamdani N (2016) Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. J Am Coll Cardio 4:312–324
van Dijk Christian GM, Oosterhuis Nynke R, Yan JX, Brandt M, Paulus Walter J, van Heerebeek L, Duncker Dirk J, Verhaar Marianne C, Fontoura D, Lourenço André P, Leite-Moreira Adelino F, Falcão-Pires I, Joles Jaap A, Cheng C (2016) Distinct endothelial cell responses in the heart and kidney microvasculature characterize the progression of heart failure with preserved ejection fraction in the obese ZSF1 rat with cardiorenal metabolic syndrome. Circulation 9:e002760
Sorop O, Heinonen I, van Kranenburg M, van de Wouw J, de Beer VJ, Nguyen ITN, Octavia Y, van Duin RWB, Stam K, van Geuns R-J, Wielopolski PA, Krestin GP, van den Meiracker AH, Verjans R, van Bilsen M, Danser AHJ, Paulus WJ, Cheng C, Linke WA, Joles JA, Verhaar MC, van der Velden J, Merkus D, Duncker DJ (2018) Multiple common comorbidities produce left ventricular diastolic dysfunction associated with coronary microvascular dysfunction, oxidative stress, and myocardial stiffening. Cardiovasc Res 114:954–964
Schwarzl M, Hamdani N, Seiler S, Alogna A, Manninger M, Reilly S, Zirngast B, Kirsch A, Steendijk P, Verderber J, Zweiker D, Eller P, Höfler G, Schauer S, Eller K, Maechler H, Pieske BM, Linke WA, Casadei B, Post H (2015) A porcine model of hypertensive cardiomyopathy: implications for heart failure with preserved ejection fraction. Am J Physiol Heart Circ Physiol 309:H1407–H1418
Hamdani N, Paulus WJ (2013) Myocardial titin and collagen in cardiac diastolic dysfunction: partners in crime. Circulation 128:5–8
van Heerebeek L, Franssen C, Hamdani N, Verheugt F, Somsen G, Paulus W (2012) Molecular and cellular basis for diastolic dysfunction. Curr Heart Fail Rep 9:293–302
Bishu K, Hamdani N, Mohammed SF, Kruger M, Ohtani T, Ogut O, Brozovich FV, Burnett JC, Linke WA, Redfield MM (2011) Sildenafil and B-type natriuretic peptide acutely phosphorylate titin and improve diastolic distensibility in vivo. Circulation 124:2882–2891
Hamdani N, Bishu KG, von Frieling-Salewsky M, Redfield MM, Linke WA (2013) Deranged myofilament phosphorylation and function in experimental heart failure with preserved ejection fraction. Cardiovasc Res 97:464–471
Waddingham MT, Sonobe T, Tsuchimochi H, Edgley AJ, Sukumaran V, Chen YC, Hansra SS, Schwenke DO, Umetani K, Aoyama K, Yagi N, Kelly DJ, Gaderi S, Herwig M, Kolijn D, Mügge A, Paulus WJ, Ogo T, Shirai M, Hamdani N, Pearson JT (2019) Diastolic dysfunction is initiated by cardiomyocyte impairment ahead of endothelial dysfunction due to increased oxidative stress and inflammation in an experimental prediabetes model. J Mol Cell Cardio 137:119–131
Alegre-Cebollada J, Kosuri P, Giganti D, Eckels E, Rivas-Pardo JA, Hamdani N, Warren CM, Solaro RJ, Linke WA, Fernández JM (2014) S-glutathionylation of cryptic cysteines enhances titin elasticity by blocking protein folding. Cell 156:1235–1246
Murdoch CE, Chaubey S, Zeng L, Yu B, Ivetic A, Walker SJ, Vanhoutte D, Heymans S, Grieve DJ, Cave AC, Brewer AC, Zhang M, Shah AM (2014) Endothelial NADPH oxidase-2 promotes interstitial cardiac fibrosis and diastolic dysfunction through proinflammatory effects and endothelial-mesenchymal transition. J Am Coll Cardio 63:2734–2741
Leite S, Cerqueira RJ, Ibarrola J, Fontoura D, Fernández-Celis A, Zannad F, Falcão-Pires I, Paulus WJ, Leite-Moreira AF, Rossignol P, López-Andrés N, Lourenço AP (2019) Arterial remodeling and dysfunction in the ZSF1 rat model of heart failure with preserved ejection fraction. Circulation 12(7):e005596
van Heerebeek L, Hamdani N, Falcão-Pires I, Leite-Moreira Adelino F, Begieneman Mark PV, Bronzwaer Jean GF, van der Velden J, Stienen Ger JM, Laarman Gerrit J, Somsen A, Verheugt Freek WA, Niessen Hans WM, Paulus Walter J (2012) Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation 126:830–839
Borbély A, van der Velden J, Papp Z, Bronzwaer JGF, Edes I, Stienen GJM, Paulus WJ (2005) Cardiomyocyte stiffness in diastolic heart failure. Circulation 111:774–781
Mohammed Selma F, Hussain S, Mirzoyev Sultan A, Edwards William D, Maleszewski Joseph J, Redfield Margaret M (2015) Coronary microvascular rarefaction and myocardial fibrosis in heart failure with preserved ejection fraction. Circulation 131:550–559
Su M-YM, Lin L-Y, Tseng Y-HE, Chang C-C, Wu C-K, Lin J-L, Tseng W-YI (2014) CMR-verified diffuse myocardial fibrosis is associated with diastolic dysfunction in HFpEF. J Am Coll Cardio 7:991–997
Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss H-P, Tschöpe C (2011) Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross-linking in heart failure and normal ejection fraction. J Am Coll Cardio 57:977–985
Falcão-Pires I, Hamdani N, Borbély A, Gavina C, Schalkwijk Casper G, van der Velden J, van Heerebeek L, Stienen Ger JM, Niessen Hans WM, Leite-Moreira Adelino F, Paulus Walter J (2011) Diabetes mellitus worsens diastolic left ventricular dysfunction in aortic stenosis through altered myocardial structure and cardiomyocyte stiffness. Circulation 124:1151–1159
López B, Querejeta R, González A, Larman M, Díez J (2012) Collagen cross-linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure: potential. Hypertension 60:677–683
Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CSP, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O’Connor CM (2014) Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardio 64:2281–2293
Shah Sanjiv J, Katz Daniel H, Selvaraj S, Burke Michael A, Yancy Clyde W, Gheorghiade M, Bonow Robert O, Huang C-C, Deo Rahul C (2015) Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation 131:269–279
Tian R, Ingwall JS (1996) Energetic basis for reduced contractile reserve in isolated rat hearts. Am J Phys 270:H1207–H1216
Sequeira V, Najafi A, McConnell M, Fowler ED, Bollen IAE, Wüst RCI, dos Remedios C, Helmes M, White E, Stienen GJM, Tardiff J, Kuster DWD, van der Velden J (2015) Synergistic role of ADP and Ca2+ in diastolic myocardial stiffness. J Physiol 593:3899–3916
Tian R, Nascimben L, Ingwall JS, Lorell BH (1997) Failure to maintain a low ADP concentration impairs diastolic function in hypertrophied rat hearts. Circulation 96:1313–1319
Luptak I, Sverdlov AL, Panagia M, Qin F, Pimentel DR, Croteau D, Siwik DA, Ingwall JS, Bachschmid MM, Balschi JA, Colucci WS (2018) Decreased ATP production and myocardial contractile reserve in metabolic heart disease. J Mol Cell Cardio 116:106–114
Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG (2006) Altered creatine kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 114:1151–1158
Sano S, Wang Y, Walsh K (2018) Clonal hematopoiesis and its impact on cardiovascular disease. Circ J
Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, Baber U, Mehran R, Fuster V, Danesh J, Frossard P, Saleheen D, Melander O, Sukhova GK, Neuberg D, Libby P, Kathiresan S, Ebert BL (2017) Clonal hematopoiesis and risk of atherosclerotic cardiovascular disease. New Eng J Med 377:111–121
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu C-L, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AAB, Cooper MA, Andrés V, Hirschi KK, Martin KA, Walsh K (2017) Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355:842–847
Sano S, Oshima K, Wang Y, MacLauchlan S, Katanasaka Y, Sano M, Zuriaga MA, Yoshiyama M, Goukassian D, Cooper MA, Fuster JJ, Walsh K (2018) Tet2-mediated clonal hematopoiesis accelerates heart failure through a mechanism involving the IL-1β/NLRP3 inflammasome. J Am Coll Cardio 71:875–886
Sano S, Oshima K, Wang Y, Katanasaka Y, Sano M, Walsh K (2018) CRISPR-mediated gene editing to assess the roles of Tet2 and Dnmt3a in clonal hematopoiesis and cardiovascular disease. Circ Res 123:335–341
Bertero E, Canepa M, Maack C, Ameri P (2018) Linking heart failure to cancer. Circulation 138(7):735–742
Levine B, Kalman J, Mayer L, Fillit HM, Packer M (1990) Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med 323:236–241
Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D’agostino RB (2003) Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham heart study. Circulation 107:1486–1491
Nymo SH, Aukrust P, Kjekshus J, McMurray JJV, Cleland JGF, Wikstrand J, Muntendam P, Wienhues-Thelen U, Latini R, Askevold ET, Gravning J, Dahl CP, Broch K, Yndestad A, Gullestad L, Ueland T (2017) Limited added value of circulating inflammatory biomarkers in chronic heart failure. JACC Heart Fail 5:256–264
Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries Daniel L, Tang WHW, Alan HBW, Fang James C, Boxer R, Sweitzer Nancy K, Levy Wayne C, Goldberg Lee R, Jessup M, Cappola Thomas P (2011) High-sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circulation 4:180–187
Boer RA, Voors AA, Muntendam P, Gilst WH, Veldhuisen DJ (2009) Galectin-3: a novel mediator of heart failure development and progression. Eur J Heart Fail 11:811–817
Parrillo JE, Cunnion RE, Epstein SE, Parker MM, Suffredini AF, Brenner M, Schaer GL, Palmeri ST, Cannon RO, Alling D, Wittes JT, Ferrans VJ, Rodriguez ER, Fauci AS (1989) A prospective, randomized, controlled trial of prednisone for dilated cardiomyopathy. New Eng J Med 321:1061–1068
Gong K, Zhang Z, Sun X, Zhang X, Li A, Yan J, Luo Q, Gao Y, Feng Y (2006) The nonspecific anti-inflammatory therapy with methotrexate for patients with chronic heart failure. Am Heart J 151:62–68
Gullestad L, Aass H, Fjeld JG, Wikeby L, Andreassen AK, Ihlen H, Simonsen S, Kjekshus J, Nitter-Hauge S, Ueland T (2001) Immunomodulating therapy with intravenous immunoglobulin in patients with chronic heart failure. Circulation 103:220–225
Mann DL (2002) Inflammatory mediators and the failing heart: past, present, and the foreseeable future. Circ Res 91:988–998
Chung Eugene S, Packer M, Lo Kim H, Fasanmade Adedigbo A, Willerson James T (2003) Randomized, double-blind, placebo-controlled, pilot trial of infliximab, a chimeric monoclonal antibody to tumor necrosis factor-alpha, in patients with moderate-to-severe heart failure: results of the anti-TNF therapy against congestive heart failure (ATTACH) trial. Circulation 107:3133–3140
Van Tassell BW, Arena R, Biondi-Zoccai G, McNair Canada J, Oddi C, Abouzaki NA, Jahangiri A, Falcao RA, Kontos MC, Shah KB, Voelkel NF, Dinarello CA, Abbate A (2014) Effects of interleukin-1 blockade with anakinra on aerobic exercise capacity in patients with heart failure and preserved ejection fraction (from the D-HART pilot study). Am J Cardio 113:321–327
Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ (2017) Antiinflammatory therapy with canakinumab for atherosclerotic disease. New Eng J Med 377:1119–1131
Everett Brendan M, Cornel J, Lainscak M, Anker Stefan D, Abbate A, Thuren T, Libby P, Glynn Robert J, Ridker Paul M (2018) Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation
Juni RP, Kuster DWD, Goebel M, Helmes M, Musters RJP, van der Velden J, Koolwijk P, Paulus WJ, van Hinsbergh VWM (2019) Cardiac microvascular endothelial enhancement of cardiomyocyte function is impaired by inflammation and restored by empagliflozin. J Am Coll Cardio 4(5):575–591
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schiattarella, G.G., Sequeira, V. & Ameri, P. Distinctive patterns of inflammation across the heart failure syndrome. Heart Fail Rev 26, 1333–1344 (2021). https://doi.org/10.1007/s10741-020-09949-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10741-020-09949-5