Abstract
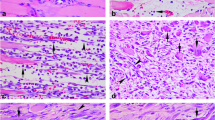
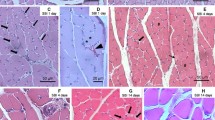
Recent studies have shown that early growth response factor-1 (Egr-1) plays an important role in regulation of inflammation and tissue repair, but little is known about its expression after trauma to skeletal muscles. A preliminary study on time-dependent expression and distribution of Egr-1 was performed by immunohistochemistry, immunofluorescence and Western blotting during skeletal muscle wound healing in rats. An animal model of skeletal muscle contusion was established in 45 Sprague-Dawley male rats. Samples were taken at 6 h, 12 h, 1 day, 3 days, 5 days, 7 days, 10 days, 14 days and 21 days post-injury, respectively (5 rats in each posttraumatic interval). 5 rats were employed as control. In the uninjured controls, Egr-1 positive staining was observed in the sarcoplasm and nuclei of normal myofibers. In wounded specimens, a small number of polymorphonuclear cells (PMNs), a number of mononuclear cells (MNCs), fibroblastic cells (FBCs) and regenerated multinucleated myotubes showed positive reaction for Egr-1 in contused zones. By morphometric analysis, an increase in Egr-1 expression was verified at inflammatory phase after contusion, which reached a peak in the regenerated phase overlapping with the fibrotic phase during skeletal muscle wound healing. The expression tendency was further confirmed by Western blotting assay. By immunofluorescent staining for co-localization, the Egr-1-positive MNCs and FBCs in wounds were identified as macrophages and myofibroblasts. The results demonstrate that the expression of Egr-1 is up-regulated and temporally distributed in certain cell types after trauma to skeletal muscles, which may be closely involved in inflammatory response, fibrotic repair and muscle regeneration during skeletal muscle wound healing.





Similar content being viewed by others
References
Bae SK, Bae MH, Ahn MY et al (1999) Egr-1 mediates transcriptional activation of IGF-II gene in response to hypoxia. Cancer Res 59:5989–5994
Baum CL, Arpey CJ (2005) Normal cutaneous wound healing: clinical correlation with cellular and molecular events. Dermatol Surg 31:674–686
Bhattacharyya S, Chen SJ, Wu M et al (2008) Smad-independent transforming growth factor-beta regulation of early growth response-1 and sustained expression in fibrosis: implications for scleroderma. Am J Pathol 173:1085–1099
Chargé SB, Rudnicki MA (2004) Cellular and molecular regulation of muscle regeneration. Physiol Rev 84:209–238
Chen SJ, Ning H, Ishida W et al (2006) The early-immediate gene EGR-1 is induced by transforming growth factor-beta and mediates stimulation of collagen gene expression. J Biol Chem 281:21183–21197
Ducruet AF, Sosunov SA, Visovatti SH et al (2011) Paradoxical exacerbation of neuronal injury in reperfused stroke despite improved blood flow and reduced inflammation in early growth response-1 gene-deleted mice. Neurol Res 33:717–725
Filippin LI, Cuevas MJ, Lima E et al (2011) Nitric oxide regulates the repair of injured skeletal muscle. Nitric Oxide 24:43–49
Florini JR, Ewton DZ, Coolican SA (1996) Growth hormone and the insulin-like growth factor system in myogenesis. Endocr Rev 17:481–517
Gashler A, Sukhatme VP (1995) Early growth response protein 1 (Egr-1): prototype of a zinc-finger family of transcription factors. Prog Nucleic Acid Res Mol Biol 50:191–224
Houston P, Campbell CJ, Svaren J et al (2001) The transcriptional corepressor NAB2 blocks Egr-1-mediated growth factor activation and angiogenesis. Biochem Biophys Res Commun 283:480–486
Karalaki M, Fili S, Philippou A et al (2009) Muscle regeneration: cellular and molecular events. In Vivo 23:779–796
Khachigian LM (2006) Early growth response-1 in cardiovascular pathobiology. Circ Res 98:186–191
Li Y, Huard J (2002) Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol 161:895–907
Ngiam N, Post M, Kavanagh BP (2007) Early growth response factor-1 in acute lung injury. Am J Physiol Lung Cell Mol Physiol 293:L1089–L1091
Pagel JI, Deindl E (2011) Early growth response 1–a transcription factor in the crossfire of signal transduction cascades. Indian J Biochem Biophys 48:226–235
Pawlinski R, Pedersen B, Kehrle B et al (2003) Regulation of tissue factor and inflammatory mediators by Egr-1 in a mouse endotoxemia model. Blood 101:3940–3947
Prisk V, Huard J (2003) Muscle injuries and repair: the role of prostaglandins and inflammation. Histol Histopathol 18:1243–1256
Pritchard MT, Nagy LE (2005) Ethanol-induced liver injury: potential roles for egr-1. Alcohol Clin Exp Res 29:146S–150S
Schmidt J, Stoffels B, Moore BA et al (2008) Proinflammatory role of leukocyte-derived Egr-1 in the development of murine postoperative ileus. Gastroenterology 135:926–936
Sheehan SM, Tatsumi R, Temm-Grove CJ et al (2000) HGF is an autocrine growth factor for skeletal muscle satellite cells in vitro. Muscle Nerve 23:239–245
Skorokhod A, Bachmann J, Giese N et al (2012) Real-imaging cDNA-AFLP transcript profiling of pancreatic cancer patients: Egr-1 as a potential key regulator of muscle Cachexia. BMC Cancer 12:265
Tanaka Y, Yamaguchi A, Fujikawa T et al (2008) Expression of mRNA for specific fibroblast growth factors associates with that of the myogenic markers MyoD and proliferating cell nuclear antigen in regenerating and overloaded rat plantaris muscle. Acta Physiol (Oxf) 94:149–159
Thiel G, Cibelli G (2002) Regulation of life and death by the zinc finger transcription factor Egr-1. J Cell Physiol 193:287–292
Wu M, Melichian DS, de la Garza M et al (2009) Essential roles for early growth response transcription factor Egr-1 in tissue fibrosis and wound healing. Am J Pathol 175:1041–1055
Yamada M, Sankoda Y, Tatsumi R et al (2008) Matrix metalloproteinase-2 mediates stretch-induced activation of skeletal muscle satellite cells in a nitric oxide-dependent manner. Int J Biochem Cell Biol 40:2183–2191
Yan SF, Zou YS, Gao Y et al (1998) Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia. Proc Natl Acad Sci USA 95:8298–8303
Yan SF, Fujita T, Lu J et al (2000a) Egr-1, a master switch coordinating up-regulation of divergent gene families underlying ischemic stress. Nat Med 6:1355–1361
Yan SF, Lu J, Xu L et al (2000b) Pulmonary expression of early growth response-1: biphasic time course and effect of oxygen concentration. J Appl Physiol 88:2303–2309
Yu TS, Cheng ZH, Li LQ et al (2010) The cannabinoid receptor type 2 is time-dependently expressed during skeletal muscle wound healing in rats. Int J Legal Med 124:397–404
Acknowledgments
This study was financially supported by grants from Research Fund for the Doctoral Program funded by Wenzhou Medical College (89212001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, YY., Ye, GH., Lin, KZ. et al. Time-dependent expression and distribution of Egr-1 during skeletal muscle wound healing in rats. J Mol Hist 44, 75–81 (2013). https://doi.org/10.1007/s10735-012-9445-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10735-012-9445-8