Abstract
The influence of nickel (Ni) and/or selenium (Se) on phospholipid composition was studied in shoots and roots of wheat seedlings. Phospholipid differences between samples were analysed using liquid chromatography/electrospray ionization–MS coupled. A total of 39 lipid species were identified. Individual phospholipids were then quantified using a multiple reaction monitoring method. In the roots, Ni toxicity was associated with an elevated level of phosphatidic acid species. In the shoots, the phosphatidylcholine/phosphatidylethanolamine ratio was about fivefold higher than in roots and decreased in Ni-treated samples. Additionally, the concentrations of phospholipid species containing C 18:3 fatty acid were reduced. Lipidome data were then analyzed using principal component analysis, which confirmed the compositional changes in phospholipids in response to Ni and Ni + Se. In contrast, the phospholipid profiles of wheat seedlings exposed to Se alone showed more similarities with the control. Together, our results suggested that the presence of Se, despite a considerable improvement of growth of Ni-treated wheat, did not counterbalance negative effect of Ni on the phospholipid composition in wheat roots and shoots.
Similar content being viewed by others
Introduction
Nickel (Ni) is a common co-contaminant in soils and sediments due to anthropogenic pollution (e.g. mining, refining, metal plating) and natural emissions (van Nostrand et al. 2008). In recent years, Ni pollution has been reported from across the world, including Asia, Europe and North America (Chen et al. 2009).
Very low concentrations of Ni are essential for plants, however, there are reports presenting toxic effects of high concentrations of this heavy metal on plants (Chen et al. 2009). Although influence of Ni on plants has been studied by many researchers, the mechanisms of its toxicity are not yet fully understood. It is considered that phytotoxicity of Ni is associated with disturbances in different physiological and metabolic processes. In our previous papers we documented that exposure of wheat seedlings to Ni resulted in oxidative stress and changes in nitrogen and carbon metabolism (Gajewska and Skłodowska 2007, 2009; Gajewska et al. 2013). Recently we reported Ni-induced increase in membrane permeability and alterations in the total fatty acid composition, leading to a decrease in their unsaturation, which was indicated by a reduction in the double bond index (Gajewska et al. 2012). To expand our knowledge on Ni toxicity to the cell membrane, in the present work we examined the influence of the metal on wheat phospholipids.
Selenium (Se) is not considered as an essential nutrient for plants, however at low doses has been shown to promote their growth. The beneficial effect of Se on plants is usually ascribed to its ability to enhance antioxidative capacity of cells (Xue et al. 2001). Nevertheless, at excess concentrations this metalloid is toxic to plants, which is related to the competition between Se and sulphur and subsequent production of proteins with disturbed function and structure (Hasanuzzaman et al. 2010).
There is some evidence that Se may increase tolerance of plants to different abiotic stress factors including heavy metals (Filek et al. 2008; Hasanuzzaman et al. 2012). Therefore, the aim of our work was to check the ability of Se to alleviate Ni stress in wheat seedlings with special attention to the effect of both elements on phospholipid composition.
Materials and methods
Chemicals
1,2-Dimyristoyl-sn-glycero-3-phospho-rac-(1-glycerol) (sodium salt) [14:0/14:0 phosphatidylglycerol (PG)], 1,2-dilauroyl-sn-glycero-3-phosphoethanolamine [12:0/12:0 phosphatidylethanolamine (PE)], 1,2-dimyristoyl-sn-glycero-3-phosphocholine [14:0/14:0 phosphatidylcholine (PC)], L-R-phosphatidylinositol (PI) sodium salt from bovine liver, 1,2-dimyristoyl-sn-glycero-3-phospho-L-serine sodium salt [14: 0/14:0 (PS)] and 1,2-dimyristoyl-sn-glycero-3-phosphate (sodium salt) [14:0/14:0 phosphatidic acid (PA)] were purchased from Avanti Polar Lipids. The other chemicals were of HPLC grade from POCh (Poland).
Plant material and growth conditions
Wheat (Triticum aestivum L., cv. Zyta) seedlings were grown hydroponically in the diluted (1:4) Hoagland nutrient solution (pH 5.8) containing 50 μM Ni (NiSO4) and 15 μM Se (Na2SeO3) supplied individually or simultaneously. The seedlings grown in the medium without an addition of Ni and Se were considered as the control. The seedlings were grown in a controlled climate room at 24 °C and 175 μmol m−2 s−1 photosynthetic photon flux density (PPFD), with a 16-h photoperiod. After 7 days shoots and roots were harvested and growth parameters (total lengths), Ni and Se concentrations and the phospholipid profile were assayed.
Estimation of Ni and Se concentrations
For determination of Ni and Se concentrations samples of shoots and roots were wet digested in the mixture of 70 % HNO3 and 30 % H2O2 (5:1, v/v) using a microwave mineralizator. Nickel concentration was determined by atomic absorption spectrometry using a Varian SpectrAA 300 spectrometer (Varian Australia Pty. Ldt., Australia) at 232 nm. Selenium concentration was estimated by the method of Watkinson (1966) based on the measurement of fluorescence of cyclohexane-extracted selenite-2,3-diaminonaphthalene complex at 370/520 nm (excitation/emission) using a Hitachi fluorescence F-2500 spectrometer (Hitachi Ldt., Japan). Prior to Se analysis, Se6+ present in the samples was reduced to Se4+ by boiling of the mineralizates with an addition of 6 M HCl for 1.5 h.
Lipid extraction protocol
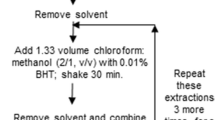
Lipids were extracted from three independent biological replicates of Triticum aestivum shoots and roots according to the method of Hummel et al. (2011) with modifications. Wheat shoots and roots (~0.5 g) were crushed in a FastPrep-24 Instrument (MP Biomedicals) homogeniser with 4 ml of the homogenous methanol:methyl-tert-butyl-ether (1:3) mixture, with 0.01 % butylated hydroxytoluene (BHT) and 100 μg ml−1 PE 24:0 as internal standard. The crushed organs were stored overnight at room temperature (RT), incubated in an ultrasonication bath at RT (10 min) and centrifuged at 1,000g for 10 min at 4 °C. After adding 2 ml of the water:methanol (3:1) mixture, the homogenate was vortexed and centrifuged at 1,000g for 10 min at 4 °C. The organic phase was transferred and evaporated. The residue was then redissolved in 1 ml of methanol/CHCl3 (4:1, v/v) and stored at −20 °C pending analysis.
HPLC–MS separation of phospholipid classes and characterisation of phospholipid molecular species
An HP1200 series HPLC system (Agilent Technologies) was used. The LC separation was performed on a Zorbax Eclipise XDB-C18 column (50 mm × 4.6 mm, 1.8 μm particle size; Agilent) at 35 °C. The total flow rate of the mobile phases (solvent mixtures A and B) was 0.6 ml min−1. The gradient started at 80 % B and 20 % A, at 2 min it linearly increased to 100 % B, held for 14 min, then in 2 min linearly decreased to 20 % B. The mobile phase consisted of 5 mM ammonium formate in water (A) and 5 mM ammonium formate in methanol (B). Prior to the use of the column, a blank gradient was run. The mass spectrometric detection was conducted on a 3200 QTRAP LC/MS/MS system from AbSciex (USA) equipped with a turbo ion spray source. MS analysis of phospholipids was performed using ESI in a negative ion mode. Nitrogen was used as curtain gas, nebulising gas, and turbo gas. The instrumental settings were as follows: spray voltage −4,500 V; curtain gas (CUR) 25; nebulizer gas (GS1) 50; turbo gas (GS2) 60; and ion source temperature 500 °C. Data analysis was performed with Analyst™ v1.5.1 software (ABSciex). To perform the phospholipid species survey, an information-dependent acquisition (IDA) method, PI (precursor ion scanning) → EPI (enhanced product ion), was used. The spectra were obtained over a range from m/z 100 to 950. The EPI scan rate was 1,000 amu s−1. A scan of the precursor for m/z 153 was used to detect the subspecies of PA, PE, PI, PS, and scan of the precursor for m/z 168 was performed to find PC.
Based on the precursor ion analysis of head groups and fatty acyls, a comprehensive list of MRM (multiple reaction monitoring) transitions was then generated to follow fatty acyl compositions of these lipids (parent R fatty acyl fragment transitions).
The relative quantifications of PLs were performed using 14:0/14:0 PE (10 μg ml−1) as an internal standard before the chromatographic determination step.
Data analysis
The percentage of lipids was expressed as the mean ± SD. Significant differences between the means were assessed using the Student’s t test. Differences at P < 0.05 were considered significant. Principal component analysis (PCA) was used to compare multivariate data from roots and shoots phospholipid profiles in Ni and/or Se presence (Markerview v1.2.1 software, ABSciex). To minimize the influence of different levels on different lipid groups, each value for a certain species of PLs was divided by the total amount of the specific group of PLs.
Results and discussion
Growth and element concentration
Exposure of wheat seedlings to Ni reduced the total shoot and root lengths by 23 and 50 % compared to the control, respectively (Table 1). Treatment of wheat seedlings with Se alone did not significantly influence the shoot growth, however it led to a 12 % reduction in the total root length. Compared to the seedlings subjected only to Ni, in those treated simultaneously with Ni and Se an improvement of the total shoot length by approximately 26 % was observed. Application of Se to the Ni-containing medium resulted also in an increase in the total root length by 68 %, compared to the seedlings treated only with Ni. In line with our results, Se-induced growth improvement has previously been reported for plants subjected to Ni (Hawrylak et al. 2007) and Cd (Filek et al. 2008) stress.
Exposure to Ni led to considerable accumulation of this metal in wheat tissues, mostly in the roots which contained about 94 % of the total amount of the metal taken up by the seedlings (Table 1). Nickel stress did not influence Se uptake by the seedlings, however addition of Se to Ni-containing medium significantly reduced accumulation of Ni both in shoots and roots, by 32 and 46 %, respectively, compared to the seedlings exposed only to Ni. Similarly to our results, a reduction in heavy metal uptake due to Se supplementation was reported by other authors (Shanker et al. 1996; He et al. 2004; Filek et al. 2008). Shanker et al. (1996) suggested that limitation of heavy metal uptake by Se might be related to the reduction of the metal availability for plants due to the formation of non toxic Se-metal complexes.
Characterisation of phospholipid molecular species
Tandem MS/MS analysis of phospholipid species in a negative ion mode reveals structural information on fatty acid chains at sn-1 and sn-2 position of the phospholipid. Therefore, MS/MS experiments were performed to confirm the identities of two fatty acid chains of each species in all detected phospholipid classes. Table 2 shows molecular species in each phospholipid class identified in the roots and shoots of wheat seedlings. Using the LC/ESI–MS/MS procedure, 39 species of PLs were identified, and the number of identified species for PA, PC, PE, PG, PI, PS were 9, 7, 8, 3, 3 and 9, respectively. Further quantitative analysis (Table 3) of the major PLs for both organs revealed that the total amount of phospholipids was the lowest in the shoots of wheat seedlings treated with Ni both used individually and in combination with Se. Also, significant decreases in the content of phospholipid classes were reported in cadmium stressed tomato (Ouariti et al. 1997; Ben Ammar et al. 2007).
Figure 1 shows the composition of PLs for wheat seedlings in Ni and/or Se presence. In the roots and shoots, PC was the predominant PL. Other determined PLs classes were PE, PI, PA, PG and PS. The same PLs classes were described by Pereyra et al. (2006) in Triticum aestivum roots. Overall, the amounts of different groups of PLs showed differences between roots and shoots. In the roots, the dominant species were PC and PE in control samples accounting for 32 and 30 %, respectively, whereas in the shoots the PC class was about 62 %. Statistically significant differences, were most often, observed in the PA class. PA constituted around 18 and 3 % of total cell phospholipids in control samples of roots and shoots, respectively. When comparing control samples with those exposed to the elements, relatively high amount of PA was found in the roots exposed to Ni + Se. PA level significantly increased also in Ni and/or Se treated shoots. Normally, PA constitutes only a minor proportion of the cellular lipid pool but, in plants, in response to different conditions of stress factors, such as bacterial and fungal pathogens, H2O2, chilling, salts, wounding, heavy metals exposure, PA levels can increase significantly (Testerink and Munnik 2005; Darwish et al. 2009). In the wheat shoots, significant increases in PA species were accompanied by a lower level of PC. These results suggest possible conversion of PC species to PA via a phospholipase D (PLD) pathway (Testerink and Munnik 2005).
Comparison of phospholipid composition of wheat roots and shoots exposed to Ni and/or Se. PA phosphatidic acid, PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol, PG phosphatidylglycerol, PS phosphatidylserine; *, ** indicate values that differ significantly from the control at P < 0.05 and P < 0.01, respectively
Phosphatidylcholine and phosphatidylethanolamine are the two major membrane components of both prokaryotic and eukaryotic cells (Sutoh et al. 2010). PC forms a bilayer structure, whereas PE molecules are nonbilayer PLs (Mansour et al. 2002). In addition, a high level of choline in the polar head group has a fluidizing effect on the membrane, whereas ethanolamine makes the membrane more rigid (Quartacci et al. 2001). Thus, the relative compositional change of phospholipid species in living organisms resulted in the modifications of the PC/PE ratio (Quartacci et al. 2001). In our study, the ratio in control shoots was about fivefold higher than in roots (Fig. 2). Treatment of wheat seedlings with Ni used individually or in combination with Se induced a statistically significant increase in the PE level in the shoots (Fig. 1), which resulted in a marked decline in the PC/PE ratio. It seems that Ni presence causes lipid homeostasis disorders, possibly leading to a decrease in the membrane fluidity. A similar phenomenon was observed in wheat roots grown in copper excess, which may have led to enriched non-lamellar-forming domains and phase separation of non-lamellar-forming lipids (Quartacci et al. 2001).
PI contents in the control wheat seedlings reached 16 and 10 % in roots and shoots, respectively (Fig. 1). However, a lower content of PI was noted in the roots of wheat Triticum durum (Quartacci et al. 2001). Among the obtained samples, statistically significant increase in PI content was observed in the roots exposed to Se.
Phosphatidylglycerol is the major phospholipid in the thylakoid membranes of plant chloroplasts (Welti et al. 2003). PG is considered to be essential for growth and development of the chloroplast (Su et al. 2009). In our study, shoots contained higher amount of PG comparing to roots. However, the content of PG did not show significant alterations in wheat seedlings in the elements presence.
Phosphatidylserine was a minor lipid species, comprising less than 6 % in the shoots and trace amounts of total PLs in the roots (Fig. 1). Small amounts of PS were also described for other plants (Vance and Steenbergen 2005). Although PS is a minor phospholipid component of plant membranes, it is likely to have other functions in addition to contributing to the structure of lipid bilayers (Delhaize et al. 1999). The best-known role of PS as an enzyme cofactor is as a specific activator of the conventional isoforms of protein kinase C. An important function of PS is also serving as a precursor of PE (Vance and Steenbergen 2005). In the examined wheat seedlings, statistically significant increase was observed only in Se treated shoots.
Analysis of the distribution of the hydrocarbon chains showed that palmitic acid (C16:0) and stearic acid (C18:0) were the most predominant saturated fatty acids in both wheat organs, whereas oleic acid (C18:1), linoleic acid (C18:2) and linolenic acid (C18:3) were the major unsaturated species (Figs. 3, 4). This was in accordance with our previous work (Gajewska et al. 2012).
Relative abundances for major species of phospholipids in roots of wheat seedlings. Control (black bar), Nickel (light grey bar), Selenium (white bar) and Ni + Se (dark grey bar). PA phosphatidic acid, PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol, PG phosphatidylglycerol, PS phosphatidylserine; *, **, *** indicate values that differ significantly from the control at P < 0.05, P < 0.01 and P < 0.001, respectively
Relative abundances for major species of phospholipids in shoots of wheat seedlings. Control (black bar), Nickel (light grey bar), Selenium (white bar) and Ni + Se (dark grey bar). PA phosphatidic acid, PC phosphatidylcholine, PE phosphatidylethanolamine, PI phosphatidylinositol, PG phosphatidylglycerol, PS phosphatidylserine; *, **, *** indicate values that differ significantly from the control at P < 0.05, P < 0.01 and P < 0.001, respectively
Among the root phospholipids, Ni-treated seedlings exhibited a significant decrease in the content of phospholipid molecular species, especially, those containing C18:3 fatty acid: PA 16:0/18:3, PA 18:2/18:3, PA 18:3/18:3 and a statistically significant increase in PC 18:1/18:2, PE 16:0/18:2, PS 16:0/18:2 and PS 18:1/18:2 (Fig. 3). Similar results were obtained for Ni + Se-treated seedlings. On the other hand, Se influenced only PC 16:0/18:2 and PE 16:0/18:2.
In the shoot phospholipids statistically significant changes were found less frequently than in the roots. A decline was observed for PE 16:0/18:2 and PS 18:1/18:3 in Ni and/or Se-treated samples. Also, the amount of PC 18:1/18:2 and PG 16:0/18:3 decreased in Ni presence (Fig. 4). Among the shoot phospholipids the amount of PS 16:0/18:1 increased in seedlings exposed to the examined elements, while the level of PE 18:1/18:2, PG 16:0/18:2 and PA 16:0/18:2, PE 18:2/20:1 elevated in Ni and Se presence, respectively. Results for Ni-treated shoots are consistent with the observations concerning the influence of Cd, Pb and Zn on lipid metabolism in plants (Verdoni et al. 2001; Nouairi et al. 2006). Also, in our previous work we documented that in Ni presence the amount of C18:3 fatty acid extracted from wheat seedlings decreased significantly (Gajewska et al. 2012).
To investigate the metabolic differences in phospholipid species between wheat seedlings exposed to the elements, PCA analysis was used, based on the relative percentages of PLs (Fig. 5). There were 39 variables (species of lipids) to describe abundances of each lipid in each organ extract. Projection of the resulting sample scores for the first and second principal components, which together accounted for 87.1 % of the total sample variance, showed the similarities and differences between the samples. The greatest differences were between roots and shoots, which were separated primarily along the PC1 axis; PC1 explained 76.9 % of the variance of the dataset. Along the PC2 axis, which explained 10.2 % of the variance, were primarily separated control and Ni-treated samples. Results of PCA suggest that were considerable differences between Ni and control samples, and between roots and shoots. Moreover, the obtained results indicated that the changes of lipidome in samples treated with Ni and Ni + Se were similar. On the other hand, the differences in lipids profile caused by Se used individually were greater in shoots than in roots.
Conclusion
Our work is the first investigation of the PL fraction of shoots and roots of wheat seedlings treated with Ni and/or Se. Although Se addition to the growth medium resulted in lower uptake of Ni and improved growth of wheat seedlings, PCA analysis demonstrated that Se did not counteract the effects caused by Ni on wheat phospholipids. It can be concluded that the changes in the phospholipids profile may be considered as an indicator of Ni stress.
References
Ben Ammar W, Nouairi I, Zarrouk M, Jemal F (2007) Cadmium stress induces changes in the lipid composition and biosynthesis in tomato (Lycopersicon esculentum Mill.) leaves. Plant Growth Regul 53:75–85
Chen C, Huang D, Liu J (2009) Functions and toxicity of nickel in plants: recent advances and future prospects. Clean 37:304–313
Darwish E, Testerink C, Khalil M, El-Shihy O, Munnik T (2009) Phospholipid signaling responses in salt-stressed rice leaves. Plant Cell Physiol 50:986–997
Delhaize E, Hebb DM, Richards KD, Lin JM, Ryan PR, Gardner RC (1999) Cloning and expression of a wheat (Triticum aestivum L.) phosphatidylserine synthase cDNA. Overexpression in plants alters the composition of phospholipids. J Biol Chem 274:7082–7088
Filek M, Keskinen R, Hartikainen H, Szarejko I, Janiak A, Miszalski Z, Golda A (2008) The protective role of selenium in rape seedlings subjected to cadmium stress. J Plant Physiol 165:833–844
Gajewska E, Skłodowska M (2007) Effect of nickel on ROS content and antioxidative enzyme activities in wheat leaves. Biometals 20:27–36
Gajewska E, Skłodowska M (2009) Nickel-induced changes in nitrogen metabolism in wheat shoots. J Plant Physiol 166:1034–1044
Gajewska E, Bernat P, Długoński J, Skłodowska M (2012) Effect of nickel on membrane integrity, lipid peroxidation and fatty acid composition in wheat seedlings. J Agron Crop Sci 198:286–294
Gajewska E, Niewiadomska E, Tokarz K, Słaba M, Skłodowska M (2013) Nickel-induced changes in carbon metabolism. J Plant Physiol 170:369–377
Hasanuzzaman M, Hossain MA, Fujita M (2010) Selenium in higher plants: physiological role, antioxidant metabolism and abiotic stress tolerance. J Plant Sci 5:354–375
Hasanuzzaman M, Hossain MA, Fujita M (2012) Exogenous selenium pretreatment protects rapeseed seedlings from cadmium-induced oxidative stress by upregulating antioxidant defense and methylglyoxal detoxification systems. Biol Trace Elem Res 149:248–261
Hawrylak B, Matraszek R, Szymańska M (2007) Response of lettuce (Lactuca sativa L.) to selenium in nutrient solution contaminated with nickel. Veg Crops Res Bull 67:63–70
He PP, Lu XZ, Wang GY (2004) Effects of Se and Zn supplementation on the antagonism against Pb and Cd in vegetables. Environ Int 30:167–172
Hummel J, Segu S, Li Y, Irgang S, Jueppner J, Giavalisco P (2011) Ultra performance liquid chromatography and high resolution mass spectrometry for the analysis of plant lipids. Front Plant Sci 2:54
Mansour MMF, Al-Mutawa MM, Salama KHA, Abou Hadid AMF (2002) Effect of NaCl and polyamines on plasma membrane lipid of wheat roots. Biol Plant 45:235–239
Nouairi I, Ben Ammar W, Ben Youssef N, Ben Miled DD, Ghorbal MH, Zarrouk M (2006) Variations in membrane lipid metabolism in Brassica juncea and Brassica napus leaves as a response to cadmium exposure. J Agron 5:299–307
Ouariti O, Boussama N, Zarrouk M, Cherif A, Ghorbal MH (1997) Cadmium- and copper-induced changes in tomato membrane lipids. Phytochem 45:1343–1350
Pereyra MA, Zalazar CA, Barassi CA (2006) Root phospholipids in Azospirillum-inoculated wheat seedlings exposed to water stress. Plant Physiol Biochem 44:873–879
Quartacci MF, Cosi E, Navari-Izzo F (2001) Lipids and NADPH-dependent superoxide production in plasma membrane vesicles from roots of wheat grown under copper deficiency or excess. J Exp Bot 52:77–84
Shanker K, Mishra S, Srivastava S, Srivatava R, Dass S, Prakash S, Srivastava MM (1996) Effect of selenite and selenate on plant uptake of cadmium by maize (Zea mays). Bull Environ Contam Toxicol 56:419–424
Su MK, Bremer DJ, Jeannotte R, Welti R, Yang C (2009) Membrane lipid composition and heat tolerance in cool-season turfgrasses, including a hybrid bluegrass. J Am Soc Hort Sci 134:511–520
Sutoh K, Sanuki N, Sakaki T, Imai R (2010) Specific induction of TaAAPT1, an ER- and Golgi-localized ECPT-type aminoalcoholphosphotransferase, results in preferential accumulation of the phosphatidylethanolamine membrane phospholipid during cold acclimation in wheat. Plant Mol Biol 72:519–531
Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10:368–375
Van Nostrand JD, Arthur JM, Kilpatrick LE, Neely BA, Bertsch PM, Morris PJ (2008) Changes in protein expression in Burkholderia vietnamiensis PR1 301 at pH 5 and 7 with and without nickel. Microbiology 154:3813–3824
Vance JE, Steenbergen R (2005) Metabolism and functions of phosphatidylserine. Prog Lipid Res 44:207–234
Verdoni N, Mench M, Cassagne C, Bessoule JJ (2001) Fatty acid composition of tomato leaves as biomarkers of metal-contaminated soils. Environ Toxicol Chem 20:382–388
Watkinson JH (1966) Fluorometric determination of selenium in biological material with 2,3-diaminonaphthalene. Anal Chem 38:92–97
Welti R, Wang X, Williams TD (2003) Electrospray ionization tandem mass spectrometry scan modes for plant chloroplast lipids. Anal Biochem 314:149–152
Xue T, Hartikainen H, Piironen V (2001) Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 237:55–61
Acknowledgments
This study was supported by the University of Łódź Grants no. 506/819 and 506/802.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Bernat, P., Gajewska, E., Bernat, T. et al. Characterisation of the wheat phospholipid fraction in the presence of nickel and/or selenium. Plant Growth Regul 72, 163–170 (2014). https://doi.org/10.1007/s10725-013-9848-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9848-x