Abstract
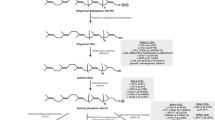
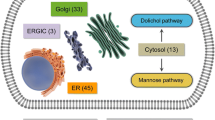
In the majority of congenital disorders of glycosylation, the assembly of the glycan precursor GlcNAc2Man9Glc3 on the polyprenol carrier dolichyl-pyrophosphate is compromised. Because N-linked glycosylation is essential to life, most types of congenital disorders of glycosylation represent partial losses of enzymatic activity. Consequently, increased availability of substrates along the glycosylation pathway can be beneficial to increase product formation by the compromised enzymes. Recently, we showed that increased dolichol availability and improved N-linked glycosylation can be achieved by inhibition of squalene biosynthesis. This review summarizes the current knowledge on the biosynthesis of dolichol-linked glycans with respect to deficiencies in N-linked glycosylation. Additionally, perspectives on therapeutic treatments targeting dolichol and dolichol-linked glycan biosynthesis are examined.

Similar content being viewed by others
References
Schwarz, F., Aebi, M.: Mechanisms and principles of N-linked protein glycosylation. Curr. Opin. Struct. Biol. 21(5), 576–582 (2011)
Larkin, A., Imperiali, B.: The expanding horizons of asparagine-linked glycosylation. Biochemistry 50(21), 4411–4426 (2011)
Kornfeld, R., Kornfeld, S.: Assembly of asparagine-linked oligosaccharides. Annu. Rev. Biochem. 54, 631–664 (1985)
Swiezewska, E., Danikiewicz, W.: Polyisoprenoids: structure, biosynthesis and function. Prog. Lipid Res. 44(4), 235–258 (2005)
Buhaescu, I., Izzedine, H.: Mevalonate pathway: a review of clinical and therapeutical implications. Clin. Biochem. 40(9–10), 575–584 (2007)
Maeda, Y., Kinoshita, T.: Dolichol-phosphate mannose synthase: structure, function and regulation. Biochim. Biophys. Acta 1780(6), 861–868 (2008)
Heesen, S., et al.: Isolation of the ALG5 locus encoding the UDP-glucose:dolichyl-phosphate glucosyltransferase from Saccharomyces cerevisiae. Eur. J. Biochem. 224(1), 71–79 (1994)
Rush, J.S., et al.: Identification and characterization of a cDNA encoding a dolichyl pyrophosphate phosphatase located in the endoplasmic reticulum of mammalian cells. J. Biol. Chem. 277(47), 45226–45234 (2002)
Rush, J.S., et al.: Recycling of dolichyl monophosphate to the cytoplasmic leaflet of the endoplasmic reticulum after the cleavage of dolichyl pyrophosphate on the lumenal monolayer. J. Biol. Chem. 283(7), 4087–4093 (2008)
van Berkel, M.A., et al.: The Saccharomyces cerevisiae CWH8 gene is required for full levels of dolichol-linked oligosaccharides in the endoplasmic reticulum and for efficient N-glycosylation. Glycobiology 9(3), 243–253 (1999)
Cantagrel, V., Lefeber, D.J.: From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J. Inherit. Metab. Dis. 34(4), 859–867 (2011)
Zelinger, L., et al.: A missense mutation in DHDDS, encoding dehydrodolichyl diphosphate synthase, is associated with autosomal-recessive retinitis pigmentosa in Ashkenazi Jews. Am. J. Hum. Genet. 88(2), 207–215 (2011)
Lefeber, D.J., et al.: Autosomal recessive dilated cardiomyopathy due to DOLK mutations results from abnormal dystroglycan O-mannosylation. PLoS Genet. 7(12), e1002427 (2011)
Stoffels, M., Simon, A.: Hyper-IgD syndrome or mevalonate kinase deficiency. Curr. Opin. Rheumatol. 23(5), 419–423 (2011)
Goldfinger, S.: The inherited autoinflammatory syndrome: a decade of discovery. Trans. Am. Clin. Climatol. Assoc. 120, 413–418 (2009)
Drenth, J.P., Haagsma, C.J., van der Meer, J.W.: Hyperimmunoglobulinemia D and periodic fever syndrome. The clinical spectrum in a series of 50 patients. International Hyper-IgD Study Group. Medicine (Baltimore) 73(3), 133–144 (1994)
Haas, D., Hoffmann, G.F.: Mevalonate kinase deficiencies: from mevalonic aciduria to hyperimmunoglobulinemia D syndrome. Orphanet J. Rare Dis. 1, 13 (2006)
Mandey, S.H., et al.: A role for geranylgeranylation in interleukin-1beta secretion. Arthritis Rheum. 54(11), 3690–3695 (2006)
Kuijk, L.M., et al.: Statin synergizes with LPS to induce IL-1beta release by THP-1 cells through activation of caspase-1. Mol. Immunol. 45(8), 2158–2165 (2008)
Hoffmann, G.F., et al.: Regulatory adaptation of isoprenoid biosynthesis and the LDL receptor pathway in fibroblasts from patients with mevalonate kinase deficiency. Pediatr. Res. 41(4 Pt 1), 541–546 (1997)
Rosenberg, T.: Epidemiology of hereditary ocular disorders. Dev. Ophthalmol. 37, 16–33 (2003)
Fliesler, S.J., Rapp, L.M., Hollyfield, J.G.: Photoreceptor-specific degeneration caused by tunicamycin. Nature 311(5986), 575–577 (1984)
Fliesler, S.J., Rayborn, M.E., Hollyfield, J.G.: Membrane morphogenesis in retinal rod outer segments: inhibition by tunicamycin. J. Cell Biol. 100(2), 574–587 (1985)
Grunewald, S.: The clinical spectrum of phosphomannomutase 2 deficiency (CDG-Ia). Biochim. Biophys. Acta 1792(9), 827–834 (2009)
Cantagrel, V., et al.: SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder. Cell 142(2), 203–217 (2010)
Kasapkara, C.S., et al.: SRD5A3-CDG: A patient with a novel mutation. Eur. J. Paediatr. Neurol. (2012)
Kranz, C., et al.: A defect in dolichol phosphate biosynthesis causes a new inherited disorder with death in early infancy. Am. J. Hum. Genet. 80(3), 433–440 (2007)
Michele, D.E., et al.: Dystroglycan matrix receptor function in cardiac myocytes is important for limiting activity-induced myocardial damage. Circ. Res. 105(10), 984–993 (2009)
Dancourt, J., et al.: A new intronic mutation in the DPM1 gene is associated with a milder form of CDG Ie in two French siblings. Pediatr. Res. 59(6), 835–839 (2006)
Garcia-Silva, M.T., et al.: Congenital disorder of glycosylation (CDG) type Ie. A new patient. J. Inherit. Metab. Dis. 27(5), 591–600 (2004)
Imbach, T., et al.: Deficiency of dolichol-phosphate-mannose synthase-1 causes congenital disorder of glycosylation type Ie. J. Clin. Invest. 105(2), 233–239 (2000)
Kim, S., et al.: Dolichol phosphate mannose synthase (DPM1) mutations define congenital disorder of glycosylation Ie (CDG-Ie). J. Clin. Invest. 105(2), 191–198 (2000)
Lefeber, D.J., et al.: Deficiency of Dol-P-Man synthase subunit DPM3 bridges the congenital disorders of glycosylation with the dystroglycanopathies. Am. J. Hum. Genet. 85(1), 76–86 (2009)
Niehues, R., et al.: Carbohydrate-deficient glycoprotein syndrome type Ib. Phosphomannose isomerase deficiency and mannose therapy. J. Clin. Invest. 101(7), 1414–1420 (1998)
Westphal, V., et al.: Genetic and metabolic analysis of the first adult with congenital disorder of glycosylation type Ib: long-term outcome and effects of mannose supplementation. Mol. Genet. Metab. 73(1), 77–85 (2001)
Marquardt, T., et al.: Correction of leukocyte adhesion deficiency type II with oral fucose. Blood 94(12), 3976–3985 (1999)
Haeuptle, M.A., et al.: Improvement of dolichol-linked oligosaccharide biosynthesis by the squalene synthase inhibitor zaragozic acid. J. Biol. Chem. 286(8), 6085–6091 (2011)
Forman, B.M., Chen, J., Evans, R.M.: Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc. Natl. Acad. Sci. U. S. A. 94(9), 4312–4317 (1997)
Abourbih, S., et al.: Effect of fibrates on lipid profiles and cardiovascular outcomes: a systematic review. Am. J. Med. 122(10), 962 e1-8 (2009)
Insel, P.A., Ostrom, R.S.: Forskolin as a tool for examining adenylyl cyclase expression, regulation, and G protein signaling. Cell. Mol. Neurobiol. 23(3), 305–314 (2003)
Konrad, M., Merz, W.E.: Regulation of N-glycosylation. Long term effect of cyclic AMP mediates enhanced synthesis of the dolichol pyrophosphate core oligosaccharide. J. Biol. Chem. 269(12), 8659–8666 (1994)
Konrad, M., Merz, W.E.: Long-term effect of cyclic AMP on N-glycosylation is caused by an increase in the activity of the cis-prenyltransferase. Biochem. J. 316(Pt 2), 575–581 (1996)
Mills, E.J., et al.: Primary prevention of cardiovascular mortality and events with statin treatments: a network meta-analysis involving more than 65,000 patients. J. Am. Coll. Cardiol. 52(22), 1769–1781 (2008)
Haeuptle, M.A., Hulsmeier, A.J., Hennet, T.: HPLC and mass spectrometry analysis of dolichol-phosphates at the cell culture scale. Anal. Biochem. 396(1), 133–138 (2010)
Low, P., et al.: Effects of mevinolin treatment on tissue dolichol and ubiquinone levels in the rat. Biochim. Biophys. Acta 1165(1), 102–109 (1992)
Charlton-Menys, V., Durrington, P.N.: Squalene synthase inhibitors: clinical pharmacology and cholesterol-lowering potential. Drugs 67(1), 11–16 (2007)
Bergstrom, J.D., et al.: Discovery, biosynthesis, and mechanism of action of the zaragozic acids: potent inhibitors of squalene synthase. Annu. Rev. Microbiol. 49, 607–639 (1995)
Baxter, A., et al.: Squalestatin 1, a potent inhibitor of squalene synthase, which lowers serum cholesterol in vivo. J. Biol. Chem. 267(17), 11705–11708 (1992)
Bergstrom, J.D., et al.: Zaragozic acids: a family of fungal metabolites that are picomolar competitive inhibitors of squalene synthase. Proc. Natl. Acad. Sci. U. S. A. 90(1), 80–84 (1993)
Chugh, A., Ray, A., Gupta, J.B.: Squalene epoxidase as hypocholesterolemic drug target revisited. Prog. Lipid Res. 42(1), 37–50 (2003)
Matzno, S., et al.: Inhibition of cholesterol biosynthesis by squalene epoxidase inhibitor avoids apoptotic cell death in L6 myoblasts. J. Lipid Res. 38(8), 1639–1648 (1997)
Horie, M., et al.: An inhibitor of squalene epoxidase, NB-598, suppresses the secretion of cholesterol and triacylglycerol and simultaneously reduces apolipoprotein B in HepG2 cells. Biochim. Biophys. Acta 1168(1), 45–51 (1993)
Abe, I., et al.: Green tea polyphenols: novel and potent inhibitors of squalene epoxidase. Biochem. Biophys. Res. Commun. 268(3), 767–771 (2000)
Wu, A.H., et al.: Effect of 2-month controlled green tea intervention on lipoprotein cholesterol, glucose, and hormonal levels in healthy postmenopausal women. Cancer. Prev. Res. (Phila), (2012)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Welti, M. Regulation of dolichol-linked glycosylation. Glycoconj J 30, 51–56 (2013). https://doi.org/10.1007/s10719-012-9417-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-012-9417-y