Abstract
As compared to cutaneous leishmaniasis, vaccination against visceral leishmaniasis (VL) has received limited attention. In this study, we demonstrate for the first time that an UDP-Galactose: N-acetylglucosamine β 1–4 galactosyltransferase (GenBank Accession No. EF159943) expressing attenuated LD clonal population (A-LD) is able to confer protection against the experimental challenge with the virulent LD AG83 parasite. A-LD was also effective in established leishmania infection. The vaccinated animals showed both cell mediated (in vitro T-cell proliferation, and DTH response) and humoral responses (Th1 type). These results demonstrate the potential of the attenuated clones as an immunotherapeutic and immunoprophylactic agent against visceral leishmaniasis.
Similar content being viewed by others
1 Introduction
Leishmaniasis is caused by parasites of the genus Leishmania, a group of kinetoplastid protozoan that are transmitted by sand flies as flagellated promastigotes. With the bite of the female vector the parasites are injected into the host to enter and multiply in the phagolysosomes of macrophages as intercellular amastigotes. At present 12 million people worldwide are infected with this dreaded parasite and 600,000 new cases are reported every year [1]. It is still a major health problem in some 88 endemic countries. Depending on the species variation there are three clinical manifestations: cutaneous, muco-cutaneous and visceral leishmaniasis (VL), of which VL is the most severe form, which is often fatal if left untreated. In India, VL, caused by Leishmania donovani, is the most prevalent form of leishmaniasis. It is estimated to affect 44 million people in 28 districts of Bihar and 5.5 million people in eight districts of West Bengal annually. In addition, association of HIV with VL as an opportunistic infection has been frequently reported. It is characterized by prolonged and irregular fever often associated with rigor and chills, splenomegaly, lymphadenopathy, hepatomegaly, pancytopenia, progressive anaemia, weight loss and hypergammaglobulinaemia with hypoalbunemia.
Conventional antileishmanial drugs [2] require long-term therapy and often induce toxic effects [3, 4]. In vitro studies have shown that Leishmania also develops resistance against miltefosine, a recently approved oral drug effective in VL treatment, by changes in or loss of a leishmanial P-type phospholipid translocase [5]. Since curative drugs leading to drug resistance cases is a major health concern, intensive efforts have been devoted to vaccine development [6–9]. Though several vaccination strategies against experimental leishmaniasis have been attempted, a successful vaccine against the disease still remains a dream. Though protein based vaccination, including gp63 and LACK, have shown promise in mice, it has usually required a strong Th1-inducing adjuvant [10, 11]. Progresses with these antigens have found limited application, because of the lack of appropriate adjuvant and inconsistent T cell response in humans [12]. Though laboratory experiments have seen success with the use of killed parasites, subunit vaccines or use of attenuated organisms as vaccine candidates, none has yet translated into an effective product [13, 14]. Studies on animals indicate that, Leishmania antigens can be presented to T cells by macrophages harboring dead organisms, but not by cells harboring live parasites. In line with this argument, vaccination with attenuated or avirulent form of leishmania, or gamma-irradiated leishmania has been shown to yield considerable protection against a virulent challenge. However, in the absence of a clear genetic profile of any avirulent organisms available, their use for human vaccination would be unacceptable, because of the risk of reversion to a virulent phenotype [15]. Our earlier work has established that the loss of virulence of LD promastigotes on longitudinal propagation was associated with the expression of a developmentally regulated galactosyltransferase (GalT4) and the stage specific expression of galactose terminal glycoconjugates in the attenuated parasite clones [16]. These attenuated clonal parasites are genetically defined and did not show any reversion to virulent phenotype when kept in culture for 30 passages. This observation impelled us to study the efficacy of attenuated LD clonal parasites as an immunoprophylactic and immunotherapeutic agent against L. donovani infection in the murine model.
2 Materials and methods
2.1 Animals, parasites and animal infection
Four to 6 week old BALB/c mice (irrespective of sex), reared in the institute (Indian Institute of Chemical Biology) facility (originally bought from Jackson Laboratory, Bar Harbour, Maine) were used, with prior approval of the animal ethics committee of the Institute. L. donovani strain AG83 (MHOM/IN/83/AG83) was maintained in golden hamsters as previously described [16]. Promastigotes obtained after transforming amastigotes from infected spleen were maintained in M199 (Invitrogen Life Technologies) as previously described [16]. For infection, 4- to 6-week-old mice were inoculated with 2 × 107, second passage promastigotes in 0.2 mL saline through the intra-cardiac route (i.c.). Splenic and hepatic parasite burden in infected animals were evaluated after Giemsa staining of the smears according to the formula of Stauber [17], and results were expressed as mean parasite number±standard deviation.
2.2 Parasite burdens
Parasites in organs were quantified by means of a culture microtitration assay as described [18]. Results were expressed as the logarithmic mean number of parasites per gram established from a minimum of five mice for each group. The detection threshold of the method is 5 × 102 parasites g−1.
2.3 Cloning of parasites by limiting dilution
Parasites from a mixed population of seventh passage were cloned by the limiting dilution method essentially as described previously [16]. Avirulent clones were selected on the basis of PNA agglutination and GalT4 expression [16].
2.4 Complete soluble antigen
Complete soluble antigen (CSA) was prepared from attenuated LD promastigotes in presence of 0.04% Non-idet P-40 essentially as described previously [19].
2.5 Macrophage erythrocyte rosettes
Kupffer cells were isolated from BALB/c mice liver [20]. Rabbit erythrocyte suspension (108cells/mL) was mixed with an equal volume of PBS containing 0.2 U of neuraminidase (Sigma) and incubated for 1 h at 37°C. Cells were washed with cold PBS and re-suspended in PBS at the original concentration. Kupffer cells (100 μL, 106) were mixed with an equal volume of neuraminidase treated rabbit erythrocytes (108cells/mL) and incubated at 22°C for 1 h. Each sample was examined without centrifugation under a phase microscope for rosette formation. In general, with strong reactions (graded as 4+) the red cells covered the entire surface of the macrophages, while in the weaker reactions (1+), fewer red cells were attached to each macrophage, and fully covered macrophages were only occasionally seen. In the rosette inhibition studies, Kupffer cells were incubated for 1 h at 37°C with four volumes of PBS containing the inhibitors (saccharide, A-LD or glycoprotein), before testing for their ability to support rosette formation.
2.6 Macrophage attachment of labeled parasites
A-LD parasites were biosynthetically labeled with 14[C] galactose and virulent LD clones (V-LD) with 3[H] mannose as previously mentioned [16]. Single cell suspension of splenocytes from BALB/c mice were prepared after Ficoll density gradient centrifugation and then suspended in complete RPMI 1640. Cells were plated in triplicate at 106 cell/cover slip. Three hours after plating, cells were incubated in presence or absence of glycosides or glycoproteins for 2 h followed by incubation with 2 × 107, 14[C] labeled A-LD or 3[H] labeled V-LD parasite suspension containing 12,000 cpm for a further period of 1 h, at 37°C. Cover slips were then washed thoroughly; cells were scrapped off using a rubber policeman and counted in a scintillation counter (Tri-Carb 2100TR; Packard Instrument).
2.7 Measurement of reactive oxygen species (RO) and nitric oxide (NO)
ROS and NO was measured essentially as described earlier [16]. Adherent macrophages (Mϕ) were incubated in presence or absence of inhibitors as mentioned above. Cells were infected with 2 × 107 clonal populations at 37°C for 6 h in 5% CO2. Free parasites were removed by repeated washings, and cultures were incubated under the same conditions for a further period of 3 days. Superoxide (O2 −) was detected by the reduction of NBT to an insoluble form (formazan). Reduction in the number of NBT+zymogen-ingested macrophages was taken as the positive control. The culture supernatants were analyzed for the presence of nitrite (NO2 −) by Griess Reagent as described before [16]. Measurement of nitrite was an indication of NO produced by these cells.
2.8 Prophylactic immunization with A-LD clonal parasites
BALB/c mice (nine in number) were immunized through the subcutaneous (s.c.) route with 107 A-LD parasites twice at 15 days interval, while control group of mice received saline. Thirty days after the last immunization, animals were challenged with 107 virulent second passage LD AG83 parasites, suspended in saline through the i.c. route. Sixty days post infection (p.i.), animals were sacrificed and hepatic and splenic parasite burden was calculated as described by Stauber [17] as well as by the limiting dilution method.
2.9 Therapeutic immunization with A-LD clonal parasites
BALB/c mice (nine in number) were infected with 107 virulent second passage LD AG83 parasites suspended in saline through the i.c route. Sixty days p.i., animals were injected twice at 15 days interval with 107 A-LD parasites through the s.c. route, while control group of mice received saline. Thirty days later animals were sacrificed and hepatic and splenic parasite burden was calculated as described by Stauber [17] as well as by the limiting dilution method.
2.10 Antileishmanial DTH response in BALB/c mice
The delayed type hypersensitivity (DTH) response was measured in BALB/c mice inoculated with AG 83 or A-LD, using CSA as an antigen according to our previously published method [21]. Animals were inoculated (i.c., 1 × 107) with the parasites. They were tested 45 days after the last injection by injecting 50 μg of CSA in the right footpad. DTH was calculated as the increase in footpad thickness at 72 h post CSA injection compared to the thickness of saline treated left footpad.
2.11 Lymphocyte proliferation assay
CSA specific T cell proliferation assay was performed as described [22]. Single cell suspension of splenocytes from different experimental groups of mice 60 days p.i. with AG83 were prepared after Ficoll density gradient centrifugation and then suspended in complete RPMI 1640. Cells were plated in triplicate at 106 cell/well concentrations in 96-well plates and allowed to proliferate for 3 days at 37°C in 5% CO2 incubator in the presence or absence of 100 μg CSA. Control cells were incubated with 3 μg/mL ConA. At 18 h before they were harvested, cells were pulsed with 1 μCi (6.7 Ci/M) [3H]thymidine/well. [3H]Thymidine uptake, as an index of proliferation, was measured by liquid scintillation counter (Tri-Carb 2100TR; Packard Instrument).
2.12 Cytokine assay
Splenic mononuclear cells (106) were cultured as above for 72 h and the supernatants were assayed for IFN-γ, IL-4, IL-10, IL-12, TNF-α and TGF-β production by ELISA according to the manufacturer’s instructions (Pharmingen).
2.13 Antibody isotype assays
Serums from different groups of mice were obtained 60 days p.i. with LD AG83 parasites and levels of parasite specific IgG1 and IgG2a were assayed by ELISA. The animals were bled by cardiac puncture and the sera were individually tested by ELISA for the presence of parasite-specific IgG1 and IgG2a antibodies. The 96 wells ELISA plates (Nunc) were coated with 1 μg/100 μL CSA, or BSA overnight at 4°C. Non-specific binding was blocked by incubation for 1 h in presence of 5% FCS in PBS at room temperature. Wells were washed extensively with blocking buffer and incubated with serial dilutions of triplicate samples of sera from various groups of mice at 37°C for 1 h, washed and re-incubated for 1 h at 37°C in presence of 100 μL alkaline phosphatase conjugated goat anti mouse IgG1 or IgG2a antibodies (1:1,000, Southern, Birmingham, AL, USA). Absorbance was read on ELISA plate reader (Multiskan MS; Labsystems) at 405 nm.
2.14 Statistical analysis
A paired two-tailed Student t-test was used for statistical analysis of the data. P values of <0.05 were considered statistically significant.
3 Results
3.1 A-LD inhibits rosette formation between Kupffer cells and asialo rabbit erythrocytes
Kupffer cells will form rosettes by binding to the terminal β 1-galactosyl residues of asialo rabbit erythrocytes. d-Galactose or saccharides with related structures and asialo glycoproteins with terminal galactose have been shown to inhibit this binding. In order to see whether this sugar specific interaction could be blocked by A-LD, Kupffer cells were incubated with A-LD prior to reaction with asialo erythrocytes. Rosetting was impaired in presence of A-LD. The galactose residues on the A-LD were involved in the inhibition of rosetting, because A-LD in which the galactose residues were either (1) blocked with galactose specific lectins or (2) were removed by galactosidase treatment, were unable to block rosetting (Table 1).
3.2 A-LD promastigotes bind to the macrophage galactose/N-acetyl galactosamine receptor
Uptake of LD parasites by macrophages constitutes the first step of pathogenesis of kala-azar. A variety of receptors for promastigotes on macrophages have been implicated. Earlier we had shown the association of an UDP-Gal:N-acetylglucosamine β 1–4 galactosyltransferase (GalT4) enzyme and a galactose terminal protein with the attenuated A-LD promastigotes [16]. The attenuated A-LD parasites were rapidly cleared from blood and were taken up by the liver [16]. To test directly for the role of the macrophage galactose/N-acetyl galactosamine receptor in binding the A-LD promastigotes, attachment of A-LD and virulent parasite clones (V-LD) to macrophage cells was measured by counting the radioactivity associated with the macrophages after allowing the radio labeled promastigotes to interact with the macrophage cells. The A-LD parasites bound to the macrophage galactose/N-acetyl galactosamine receptor. Inhibition with galactose or its derivatives, all indicated the involvement of a galactose specific receptor in the binding of A-LD to macrophages. Fifty percent inhibition was obtained at 1 ± 0.5 mM GalNAc or lactose, at 2 ± 0.5 mM melibiose or lactulose and at 2.5 ± 0.5 mM of galactose or deoxygalactose and at 100 mM concentration of asialo glycoproteins. A representative experiment of the binding inhibition studies (Fig. 1) shows that the [14C] labeled A-LD promastigotes and not 3[H] labeled V-LD promastigotes was inhibited in the presence of galactose or AAGP. The binding of A-LD decreased linearly with increasing concentrations of the inhibitors (Fig. 1).
Radiolabelled A-LD binds to murine macrophage monolayers preferentially via the macrophage galactose/N-acetylgalactosamine receptor. Kupffer cells were pre-incubated with the inhibitors (solid squares, galactose; open circles, asialo α1 acid glycoprotein) for 30 min at 22°C before the usual binding assay with either the A-LD promastigotes (solid line) or V-LD promastigotes (dotted lines). The data are the averages (±SD) from five different experiments
3.3 The leishmanicidal response to A-LD is mediated by the macrophage galactose/N-acetylgalactosamine receptor
In competitive binding experiments using d-galactose or AAGP, the very clear dose dependent decrease in parasite binding was paralleled by a dose dependent decrease in the proportion of formazan positive cells, relative to control cells (Fig. 2). With increasing concentrations of the inhibitors, there was a similar decrease in nitric oxide produced on A-LD stimulation. Incubation of the monolayer with mannose or BSA did not affect the respiratory burst, arguing against a nonspecific quench (data not shown). Further, the burst triggered by LPS is not affected by galactose or AAGP (data not shown). Hence, the reduction in the RB and NO production, observed in the competitive binding assays, indicates that the A-LD parasites entering through the Gal/GalNaAc receptor can trigger the RB.
Effects of galactose and asialo α1 acidglycoprotein (AAGP) on A-LD mediated stimulation of the respiratory burst and nitrite production. Reactive oxygen species produced in A-LD (line) treated macrophages, in presence and absence of the inhibitors, galactose (open circles) and AAGP (filled squares) were compared to LPS mediated reactive oxygen species (72% NBT+cells) produced in uninfected macrophages. Reactive nitrogen intermediates produced in A-LD (bar graph) treated macrophages, in presence and absence of the inhibitors, galactose (closed bar) and AAGP (open bar) were compared to LPS mediated NO (24 μM) production in uninfected macrophages. Level of significant variance p < 0.0001 compared with LPS stimulated macrophages
3.4 Prophylactic immunization with A-LD results in complete clearance of splenic and hepatic parasite burden following infection with L. donovani AG83. Sterile protection against leishmaniasis in BALB/c mice
A-LD immunized animals were challenged with virulent AG 83 promastigotes. All the vaccinated mice immunized with A-LD survived the lethal challenge of AG83 and remained healthy until the termination of the experiment at 8 months p.i., which was the end of our study period, whereas all nonimmunized mice succumbed to virulent LD challenge within this period (data not shown). There was complete absence of amastigotes in the impressions of stamp smears of transverse sections of spleens and livers of A-LD immunized groups (data not shown). Serial dilution assay showed complete sterile protection in nearly 80% of the animals studied with respect to their hepatic and splenic parasite burden. The rest 20% A-LD vaccinated animals that did not show sterile protection in serial dilution assay, showed >99% reduction of splenic and hepatic parasite burden when compared with respective infected controls (Fig. 3).
Sterile protection against lethal challenge of virulent strains of L. donovani in mice immunized with A-LD. Mice (nine per group) were immunized subcutaneously twice (15 days apart) with 107 A-LD parasites. Thirty days after the last immunization, the mice were infected with 1 × 107, second passage AG83 promastigotes. Organ parasite burden was determined by serial dilution assay. Results are expressed as log of total organ parasite burden. Data represents the splenic (A) and hepatic (B) parasite burden of individual animals in the protected group (open bar) and the mean±SD for nine animals per infected group (closed bar)
3.5 Therapeutic immunization with A-LD completely eliminates hepatic and splenic parasite burden
Therapeutic immunization with A-LD in 60 days infected BALB/c mice resulted in complete clearance of amastigotes in the impressions of stamp smears of transverse sections of spleens and livers of A-LD injected groups (data not shown). Serial dilution assay showed complete sterile protection in majority of the animals studied (seven of nine) with respect to their hepatic and splenic parasite burden. The rest A-LD immunized animals that did not show sterile protection in serial dilution assay, showed >99% reduction of splenic and hepatic parasite burden, when compared with respective infected controls (Fig. 4).
Therapeutic efficacy of A-LD in treatment of AG83 infected BALB/c mice. Naive age-matched L. donovani infected BALB/c mice were immunized twice at 15 days interval with 107 A-LD parasites through the s.c. route. Organ parasite burden was determined by serial dilution assay. Data represents the splenic (A) and hepatic (B) parasite burden of individual animals in the protected group (open bar) and the mean±SD for nine animals per infected group (closed bar)
3.6 Cellular immune response in A-LD immunized mice
Leishmania infection is characterized by the impairment of the cell mediated immune response. Since A-LD immunization led to complete clearance of intracellular parasite burden in the majority of the animals, the questions that we next addressed was whether A-LD immunized mice was capable of developing an immune response to leishmanial antigens. A positive DTH response towards leishmanial antigens is an indication of CMI and there are reports of an association between the DTH responses to protection. BALB/c mice were divided into 4 groups, with 9 animals in each group. Control group (I) was treated with saline, group II was infected with 107 LD AG83, while group III and IV animals were immunized with 107 A-LD clonal parasites. Thirty days later group IV was infected with 107 LD Ag83 promastigotes, while groups I through III received saline. Mice were tested 6 weeks later by injection of CSA in the right footpad. As shown (Fig. 5), AG83 infected or A-LD immunized mice hardly showed any DTH. Although failing to demonstrate any significant DTH, mice inoculated with A-LD did exhibit significant hypersensitivity after infection with LD AG83. Although A-LD immunized mice failed to show any DTH, spleen cells from these animals responded to CSA in a Lymphocyte proliferation assay (Fig. 6).
Antileishmanial DTH response in A-LD immunized BALB/c mice. I normal animals; II saline treated AG83 infected animals; III normal animals primed with A-LD; IV A-LD immunized animals infected with AG83. Six weeks after infection, the cellular immune response induced by the A-LD vaccine was evaluated by a DTH reaction at 72 h after the administration of 50 μg of CSA. *p < 0.0001 compared with respective infected control groups
Leishmania-specific T cell response. Proliferative response to CSA (1 to 100 μg) by splenocytes from A-LD immunized BALB/c mice challenged with AG83 (open triangles) and A-LD immunized normal mice (X) was compared to saline treated infected controls (filled circles). Proliferation was measured by [3H]thymidine incorporation. The results are representative of three individual experiments (n = 5/group) and data represent the mean of triplicate wells±SE (*p < 0.0001)
3.7 A-LD induces a humoral immune response with Th1 characteristic. IgG2a/IgG1 ratio as a measure of A-LD immunization induced immunity
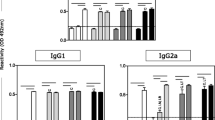
Antibody isotype switching provides a convenient surrogate marker of Th1 and Th2 CD4+ T cell differentiation [23]. IgG2a levels are dependent on IFN-γ, whereas IgG1 levels correlate with IL-4. IgG2a and IgG1 were therefore used as surrogate markers for Th1 and Th2 responses. A-LD immunization was associated with higher titers of specific IgG2a and low titers of IgG1 (Fig. 7). Using the ratio IgG2a/IgG1 as a measure of Th1:Th2 balance, A-LD immunization (3.49) was associated with a Th1 bias, whereas the LD AG83 infected ratio (0.49) revealed skewing towards Th2.
Antibody responses induced by the A-LD vaccine. Sera from A-LD immunized BALB/c mice challenged with AG83 and respective infected controls (n = 5/group) were analyzed individually for CSA-specific anti-IgG1 and anti-IgG2 Ab titers by ELISA. The results are representative of three experiments and data represent the mean±SE. IgG1 titer (A) did not vary significantly among the vaccinated groups of animals (filled squares) compared with respective infected controls (open circles), (**p < 0.0001 and *p < 0.001). IgG2 titers (B) varied significantly between the A-LD immunized (filled squares) and infected animals (open circles) (°°p < 0.0001, and °p < 0.001). Open triangles sera from normal animals
3.8 Cytokine profile in the spleen of A-LD immunized mice by ELISA
Comparison of expression of Th1 (IFN-γ, IL-12 and TNF-α) and Th2 (IL-4, IL-10 and TGF-β) cytokines in the isolated spleen cells of mice prophylactically immunized with A-LD followed by infection with LD AG83 to the cytokine profile in spleen cells of mice infected with AG83 alone indicated a Th1 bias in the A-LD immunized mice. Spleen cells isolated from A-LD immunized protected mice released higher amounts of IFN-γ, IL-12 and TNF-α as compared to AG83 infected animals. Neither the A-LD immunized nor the AG83 infected animals elicited high levels of IL-4 protein, though, IL-4 released by the AG83 infected animals was comparatively higher. Significantly high levels of IL-10 and TGF-β were released from spleen cells of AG83 infected mice as compared to A-LD vaccinated protected animals (Fig. 8).
Induction of predominant Th1 cytokine in A-LD immunized protected mice as analyzed by ELISA. Splenocytes were cultured in presence of 100 μg CSA for 72 h and supernatants were assayed by ELISA. The results are representative of three individual experiments (n = 5/group) and data represent mean (of triplicate wells) cytokine concentrations (ng/mL)±SD for IL-4, IL-10, IL-12, IFN-γ, TNF-α, and TGF-β. IL-10 and TGF-β production was decreased significantly (*p < 0.0001; **p < 0.01) for A-LD immunized mice compared with control mice whereas IFN-γ, IL-12, and TNF-α production increased significantly (*p < 0.0001) for A-LD immunized mice
4 Discussion
Considering the wealth of information on genetics and biology of the Leishmania parasite, and success of candidate vaccines in experimental models, a vaccine against different forms of leishmaniasis should be feasible. However, there is no vaccine against any form of leishmaniasis for general human use. Historically, cutaneous leishmaniasis has been the focus of vaccination attempts, probably because it has been known that individuals, who had healed their skin lesions were protected from further infections. Vaccination trials using controlled infection with live promastigotes resulted in limited success [24, 25]. Since, it is known that parasites present in skin lesions of mice are avirulent and that the avirulent organisms (which are rapidly killed by the host and provide antigens), rather than the virulent organisms, contribute most to the immune response observed in the infected mice, the use of live attenuated parasites as vaccine candidates became feasible [24]. Since, the attenuated organisms are the closest mimic to the natural course of infection, they may therefore lead to similar immune responses. It has been suggested that injection of attenuated organisms achieved better protection than any method involving recombinant proteins or subunit vaccines [24]. However, in the absence of a clear genetic profile of any avirulent cloned organisms, their use for human vaccination would have been unacceptable because of the risk of reversion to a virulent phenotype.
In the present work we have identified the galactose terminal glycoconjugates of A-LD as ligands for the Gal/GalNAc receptors on BALB/c macrophages. Our results further indicated that the binding of A-LD to the Gal/GalNAc receptor induced the macrophage microbicidal activities. Blocking of this receptor decreased this activity.
Since the A-LD clones could stimulate macrophages to produce leishmanicidal effector molecules, were stable over a long period of in vitro culture and was rapidly taken up by the liver Kupffer cells [16], we studied the vaccine potentials of this genetically defined live attenuated parasite clones. Vaccination with A-LD fully protected (sterile protection) BALB/c mice experimentally infected with virulent LD parasites. This extent of formidable protection is hitherto not achieved with any other experimentally generated attenuated parasites that have been used by the other groups [26–30].
The leishmaniases are unique among parasitic diseases, because a single vaccine has the potential to be successful at both treating and preventing the disease [11, 31, 32]. Hence, it was not surprising to note that sequential infection with the virulent parasite and the avirulent clones always resulted in a decrease in both the splenic and liver parasite burden, irrelevant of whether the virulent parasite was used for the primary or secondary infection. Therapeutic immunization with A-LD resulted in complete sterile protection in majority of the animals.
It is reasonably established that protection against Leishmaniasis is mediated principally by Th1 cells that secrete IFN-γ, a cytokine needed for activation of host macrophages that kill intracellular pathogens [33]. Thus the high IFN-γ level observed in A-LD vaccinated animals is in keeping with the state of Th1 profile that accompanies protective immunity. Though the crucial role of IFN-γ in pathogen clearance is not contested, the ability to elicit IFN-γ response cannot be the sole predictor of vaccine success. IL-12 is a potent inducer of IFN-γ that maintains memory/effector Th1 cells and is required for primary immunity to leishmaniasis [34]. There was a significantly elevated level of IL-12 production in A-LD immunized mice compared to the infected control. A-LD immunization also increased the levels of TNF-α, another inflammatory cytokine with well defined antileishmanial effects that is known to act either alone or with IFN-γ to induce the production of reactive nitrogen and oxygen intermediates [35, 36]. IL-10 and TGF-β play an important role in down regulation of the immune response [37, 38], and the presence of high levels of IL-10 and TGF-β in infected mice is in keeping with the immune suppression that is caused by VL. Experimental studies in murine models of cutaneous leishmaniasis (CL) have established a clear-cut dichotomy between Th1-mediated protection and Th2-mediated disease susceptibility [33]. The role of IL-4 as the driving Th2 cytokine responsible for disease progression has been well established in this murine model of CL [39, 40]. Its role in human disease is far less clear, though an association between VL per se and IL-4 has been suggested [41]. It has been suggested that IL-4 expression does not exert a measurable effect in murine VL as the Th1/Th2 dichotomy is less well defined in this models of VL [42–45]. Our results suggested that, though not very high, IL-4 production was higher in infected animals. The predominant Th1 immune response in vaccinated animals was further corroborated by the in vitro lymphocyte proliferation, and IgG2A antibody production. Enhanced proliferation of lymphocytes on CSA priming suggests subsequent expansion of antigen-specific T cells. Several studies have shown that Leishmania-infected murine Mϕs activate antigen-specific T cells less efficiently than uninfected cells [46], whereas protective immunity has generally been associated with a distinct cellular immune response, manifested by a strong proliferative response of peripheral blood lymphocytes to leishmanial antigens [47–49]. Lack of anti-leishmania CMI has been considered a hallmark of VL [49]. DTH reactions in the skin have been used to assess CMI in vivo. In contrast to self healing cutaneous infection, skin test responses [DTH] are conspicuously absent in patients with visceral disease and patients usually respond to parasite antigens after successful chemotherapy [50]. DTH reactivity to skin test antigens in A-LD vaccinated animals was again a positive correlate of cell mediated responses in the vaccinated animals.
The most striking result was the lack of pathogenicity of the attenuated line, which though incapable of causing disease, induced a strong antigen specific immune response, was stable over an extended period of time and was easy to produce. As a vaccine, A-LD meets the relative requirements of live-attenuated vaccines viz, (a) immunogenicity, (b) efficacy, (c) safety, (d) genetic stability and (e) ease of production and identification.
In summary, our results clearly showed that the β 1–4 GalT expressing attenuated leishmania clonal line can be used both in immunoprophylaxis and immunotherapy against visceral leishmaniasis. Our method of priming was quite distinct and novel, because it did not require any adjuvant, unlike the case for other systems. In other systems, Cyanebacterium parvum [51] or glucan [52] have been used as adjuvants with leishmanial parasites. The β 1–4 GalT expressing A-LD clone is attenuated, stable and more immunogenic than the wild type parasites. However, though there was no reversion of virulence in vivo, it is appreciated that immunization with A-LD in humans will not be possible unless more rigorous safety requirements can be met.
Abbreviations
- GalT4 UDP-galactose/N-acetylglucosamine β 1–4:
-
Galactosyltransferase
- i.c:
-
intra-cardiac
- LD:
-
Leishmania donovani
- A-LD:
-
avirulent Leishmania donovani clone
- VL:
-
visceral leishmaniasis
- s.c.:
-
sub-cutaneous
- p.i:
-
post-infection
- CMI:
-
cell mediated immunity
References
Desjeux, P.: Leishmaniasis: current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 27, 305–318 (2004)
Murray, H.W.: Clinical and experimental advances in treatment of visceral leishmaniasis. Antimicrob. Agents Chemother. 45, 2185–2197 (2001)
Croft, S.L.: Recent development in the chemotherapy of leishmaniasis. Trends Pharmacol. Sci. 9, 376–381 (1988)
Murray, H.W., Berman, J.D., Wright, S.D.: Immunochemotherapy for intracellular Leishmania donovani infection: γ-interferon plus pentavalent antimony. J. Infect. Dis. 157, 973–978 (1988)
Perez-Victoria, F.J., Castanys, S., Gamarro, F.: Leishmania donovani resistance to miltefosine involves a defective inward translocation of the drug. Antimicrob. Agents Chemother. 47, 2397–2403 (2003)
Guerin, P.J., Olliaro, P., Nosten, F., Druilhe, P., Laxminarayan, R., Binka, F., Kilama, W.L., Ford, N., White, N.J.: Malaria: current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2, 564–573 (2002)
Sundar, S., More, D.K., Singh, M.K., Singh, V.P., Sharma, S., Makharia, A., Kumar, P.C., Murray, H.W.: Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31, 1104–1107 (2000)
Grogl, M., Thomason, T.N., Franke, E.D.: Drug resistance in leishmaniasis: its implication in systemic chemotherapy of cutaneous and mucocutaneous disease. Am. J. Trop. Med. Hyg. 47, 117–126 (1992)
Jackson, J.E., Tally, J.D., Ellis, W.Y., Mebrahtu, Y.B., Lawyer, P.G., Were, J.B., Reed, S.G., Panisko, D.M., Limmer, B.L.: Quantitative in vitro drug potency and drug susceptibility evaluation of Leishmania spp. from patients unresponsive to pentavalent antimony. Am. J. Trop. Med. Hyg. 43, 464–480 (1990)
Murray, H.W., Berman, J.D., Davies, C.R., Saravia, N.G.: Advances in leishmaniasis. Lancet. 366, 1561–1577 (2005)
Coler, R.N., Reed, S.G.: Second-generation vaccines against leishmaniasis. Trends Parasitol. 21, 44–49 (2005)
Mendonca, S.C., Russell, D.G., Coutinho, S.G.: Analysis of the human T cell responsiveness to purified antigens of Leishmania: lipophosphoglycan (LPG) and glycoprotein 63 (gp 63). Clin. Exp. Immunol. 83, 472–478 (1991)
Khalil, E.A., El Hassan, A.M., Zijlstra, E.E., Mukhtar, M.M., Ghalib, H.W., Musa, B., Ibrahim, M.E., Kamil, A.A., Elsheikh, M., Babiker, A., Modabber, F.: Autoclaved Leishmania major vaccine for prevention of visceral leishmaniasis: a randomized, double-blind, BCG-controlled trial in Sudan. Lancet. 356, 1565–1569 (2000)
Sharifi, I., FeKri, A.R., Aflatonian, M.R., Khamesipour, A., Nadim, A., Mousavi, M.R., Momeni, A.Z., Dowlati, Y., Godal, T., Zicker, F., Smith, P.G., Modabber, F.: Randomized vaccine trial of single dose of killed Leishmania major plus BCG against anthroponotic cutaneous leishmaniasis in Bam, Iran. Lancet. 351, 1540–1543 (1998)
Handman, E.: Protective saliva: a novel approach to a Leishmania vaccine. Trends Parasitol. 17, 513–514 (2001)
Bhaumik, S.K., Singh, M., Basu, R., Bhaumik, S., Roychoudhury, K., Naskar, K., Roy, S., De, T.: Virulence attenuation of a UDP-galactose/N-acetylglucosamine beta1,4 galactosyltransferase expressing Leishmania donovani promastigote. Glycoconj J. 25, 459–472 (2008)
Stauber, L.A.: Host resistance to Khartoum strain of Leishmania donovani. Rice Inst. Pam. 45, 80–83 (1958)
Melby, P.C., Chandrasekar, B., Zhao, W., Coe, J.E.: The hamster as a model of human visceral leishmaniasis: progressive disease and impaired generation of nitric oxide in the face of a prominent Th1-like cytokine response. J. Immunol. 166, 1912–1920 (2001)
De, T., Roy, S.: Infectivity and attenuation of Leishmania donovani promastigotes: association of galactosyl transferase with loss of parasite virulence. J. Parasitol. 85, 54–59 (1999)
Schlepper-Schäfer, J., Kolb-Bachofen, V., Kolb, H.: Analysis of lectin-dependent recognition of desialylated erythrocytes by Kupffer cells. Biochem. J. 186, 827–831 (1980)
Mukhopadhyay, S., Bhattacharyya, S., Majhi, R., De, T., Naskar, K., Majumdar, S., Roy, S.: Use of an attenuated leishmanial parasite as an immunoprophylactic and immunotherapeutic agent against murine visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 7, 233–240 (2000)
Roy, S., Scherer, M.T., Briner, T.J., Smith, J.A., Gefter, M.L.: Murine MHC polymorphism and T cell specificities. Science. 244, 572–575 (1989)
Coffman, R.L., Lehman, D.A., Rothman, P.: Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 54, 229–270 (1993)
Handman, E.: Leishmaniasis: current status of vaccine development. Clin. Microbiol. Rev. 14, 229–243 (2001)
Khamesipour, A., Rafati, S., Davoudi, N., Maboudi, F., Modabber, F.: Leishmaniasis vaccine candidates for development: a global overview Ind. J. Med. Res. 123, 423–438 (2006)
Daneshvar, H., Coombs, G.H., Hagan, P., Phillips, R.S.: Leishmania mexicana and Leishmania major: attenuation of wild-type parasites and vaccination with the attenuated lines. J. Infect. Dis. 87, 1662–1668 (2003)
Titus, R.G., Gueiros-Filho, F.J., de Freitas, L.A., Beverley, S.M.: Development of a safe live Leishmania vaccine line by gene replacement. Proc. Natl. Acad. Sci. U. S. A. 92, 10267–10271 (1995)
Gorczynski, R.M.: Immunization of susceptible BALB/c mice against Leishmania braziliensis. I. Resistance induced using as immunogen adherent or nonadherent cells from infected mice. Cell. Immunol. 94, 1–10 (1985)
Papadopoulou, B., Roy, G., Breton, M., Kundig, C., Dumas, C., Fillion, I., Singh, A.K., Olivier, M., Ouellette, M.: Reduced infectivity of a Leishmania donovani biopterin transporter genetic mutant and its use as an attenuated strain for vaccination. Infect. Immun. 70, 62–68 (2002)
Daneshyar, H., Hagan, P., Phillips, R.S.: Leishmania mexicana H-line attenuated under pressure of gentamicin, potentiates a Th1 response and control of cutaneous leishmaniasis in BALB/c mice. Parasite Immunol. 25, 589–596 (2003)
Calvopina, M., Barroso, P.A., Marco, J.D., Korenaga, M., Cooper, P.J., Nonaka, S., Hashiguchi, Y.: Efficacy of vaccination with a combination of Leishmania amastigote antigens and the lipid A-analogue ONO-4007 for immunoprophylaxis and immunotherapy against Leishmania amazonensis infection in a murine model of New World cutaneous leishmaniasis. Vaccine. 24, 5645–5652 (2006)
Mukhopadhyay, S., Sen, P., Bhattacharyya, S., Majumdar, S., Roy, S.: Immunoprophylaxis and immunotherapy against experimental visceral leishmaniasis. Vaccine. 17, 291–300 (1999)
Liew, F.Y., O’Donnell, C.A.: Immunology of leishmaniasis. Adv. Parasitol. 32, 161–169 (1993)
Galib, H.W., Whittle, J.A., Kubin, M., Hashim, F.A., el-Hassan, A.M., Grabstein, K.H., Trinchieri, G., Reed, S.S.: IL-12 enhances Th1-type responses in human Leishmania donovani infections. J. Immunol. 154, 4623–4629 (1995)
Taylor, A., Mur, , ray, H.W.: Intracellular antimicrobial activity in the absence of interferon-γ: effect of interleukin 12 in experimental visceral leishmaniasis in interferon-γ gene-disrupted mice. J. Exp. Med. 185, 1231–1239 (1997)
Afonso, L.C., Scharton, T.M., Vieira, L.Q., Wysocka, M., Trinchieri, G., Scott, P.: The adjuvant effect of interleukin-12 in a vaccine against Leishmania major. Science. 263, 235–7 (1994)
Maloy, K.J., Powrie, F.: Regulatory T cells in the control of immune pathology. Nat. Immunol. 2, 816–822 (2001)
Maloy, K.J., Salaun, L., Cahill, R., Dougan, G., Saunders, N.J., Powrie, F.: CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 197, 111–119 (2003)
Himmelrich, H., Maillard, I., Biedermann, T., Tacchini-Cottier, F., Locksley, R.M., Rocken, M., Louis, J.A.: In BALB/c mice, IL-4 production during the initial phase of infection with Leishmania major is necessary and sufficient to instruct Th2 cell development resulting in progressive disease. J. Immunol. 164, 4819–4825 (2000)
Launois, P., Himmelrich, H., Tacchini-Cottier, F., Milon, G., Louis, J.A.: New insight into the mechanisms underlying Th2 cell development and susceptibility to Leishmania major in BALB/c mice. Microbes Infect. 1, 59–64 (1999)
Blackweell, J.M., Ibrahim, M.E., Miller, E.N., Peacock, C.S., Khalil, E.A., Cordell, H.J., Howson, J.M., El Hassan, A.M., Bereir, R.E., Blackwell, J.M.: Genes Immun. 4, 351–355 (2003)
Kropf, P., Schopf, L.R., Chung, C.L., Xu, D., Liew, F.Y., Sypek, J.P., Muller, J.P.: Expression of Th2 cytokines and the stable Th2 marker ST2L in the absence of IL-4 during Leishmania major infection. Eur. J. Immunol. 29, 3621–3628 (1999)
McMahon-Pratt, D., Alexander, J.: Does the Leishmania major paradigm of pathogenesis and protection hold for New World cutaneous leishmaniases or the visceral disease. Immunol. Rev. 201, 206–224 (2004)
Melby, P.C., Tabares, A., Restrepo, B.I., Cardona, A.E., McGruff, H.S., Teale, J.M.: Leishmania donovani: evolution and architecture of the splenic cellular immune response related to control of infection. Exp. Parasitol. 99, 17–25 (2001)
Murray, H.W., Flanders, K.C., Donaldson, D.D., Sypek, J.P., Gotwals, P.J., Liu, J., Ma, X, , .: Antagonizing deactivating cytokines to enhance host defense andchemotherapy in experimental visceral leishmaniasis. Infect. Immun. 73, 3903–3911 (2005)
Bogdan, C.: Mechanisms and consequences of persistence of intracellular pathogens: leishmaniasis as an example. Cell. Microbiol. 10, 1221–1234 (2008)
Sacks, D., Lal, S.L., Shrivastava, S.N., Blackwell, J., Neva, F.A.: An analysis of T cell responsiveness in Indian kala-azar. J. Immunol. 138, 908–913 (1987)
Pinelli, E., Killick-Kendrick, R., Wagenaar, J., Bernadina, W., del Real, G., Ruitenberg, J.: Cellular and humoral immune responses in dogs experimentally and naturally infected with Leishmania infantum. Infect. Immun. 62, 229–235 (1994)
Gazzinelli, R.T., Talvani, A., Camargo, M.M., Santiago, H.C., Oliveira, M.A., Vieira, L.Q., Martins, G.A., Aliberti, J.C., Silva, J.S.: Induction of cell-mediated immunity during early stages of infection with intracellular protozoa. Braz. J. Med. Biol. Res. 31, 89–104 (1998)
Sundar, S., Rai, M.: Laboratory diagnosis of visceral leishmaniasis. Clin. Diag. Lab. Immunol. 9, 951–958 (2002)
Hamndman, E.: Leishmania vaccine: old and new. Parasitol. Today. 13, 236–238 (1997)
Holbrook, T.W., Cook, J.A., Parker, E.W.: Immunization against Leishmania donovani: glucan as an adjuvant with killed promastigotes. Am. J. Trop. Med. Hyg. 30, 762–768 (1981)
Acknowledgements
This work was supported by the Department of Science and Technology, Government of India (grant numbers, SP/SO/B-04/2000 AND SR/S0/HS-46/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bhaumik, S.K., Singh, M.K., Karmakar, S. et al. UDP-Gal: N-acetylglucosamine β 1–4 galactosyltransferase expressing live attenuated parasites as vaccine for visceral leishmaniasis. Glycoconj J 26, 663–673 (2009). https://doi.org/10.1007/s10719-008-9212-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10719-008-9212-y