Abstract
Soil nitrous oxide (N2O) fluxes comprise a significant part of the greenhouse gas emissions of agricultural products but are spatially and temporally variable, due to complex interactions between climate, soil and management variables. This study aimed to identify the main factors that affect N2O emissions under sugarcane, using a multi-site database from field experiments. Greenhouse gas fluxes, soil, climate, and management data were obtained from 13 field trials spanning the 2011–2017 period. We conducted exploratory, descriptive and inferential data analyses in experiments with varying fertiliser and stillage (vinasse) type and rate, and crop residue rates. The most relevant period of high N2O fluxes was the first 46 days after fertiliser application. The results indicate a strong positive correlation of cumulative N2O with nitrogen (N) fertiliser rate, soil fungi community (18S rRNA gene), soil ammonium (NH4+) and nitrate (NO3−); and a moderate negative correlation with amoA genes of ammonia-oxidising archaea (AOA) and soil organic matter content. The regression analysis revealed that easily routinely measured climate and management-related variables explained over 50% of the variation in cumulative N2O emissions, and that additional soil chemical and physical parameters improved the regression fit with an R2 = 0.65. Cross-wavelet analysis indicated significant correlations of N2O fluxes with rainfall and air temperature up to 64 days, associated with temporal lags of 2 to 4 days in some experiments, and presenting a good environmental control over fluxes in general. The nitrogen fertiliser mean emission factors ranged from 0.03 to 1.17% of N applied, with urea and ammonium nitrate plus vinasse producing high emissions, while ammonium sulphate, ammonium nitrate without vinasse, calcium nitrate, and mitigation alternatives (nitrification inhibitors and timing of vinasse application) producing low N2O-EFs. Measurements from multiple sites spanning several cropping seasons were useful for exploring the influence of environmental and management-related variables on soil N2O emissions in sugarcane production, providing support for global warming mitigation strategies, nitrogen management policies, and increased agricultural input efficiency.
Similar content being viewed by others
Introduction
Global human-induced nitrous oxide (N2O) emissions have increased by 30% over the past four decades, mostly from nitrogen fertiliser application on cropland (Tian et al. 2020). Brazil and other emerging economies are responsible for a large share of these increased emissions. Sugarcane is an important crop globally, with close to 26.8 Mha harvested in over 100 countries, with Brazil accounting for over a third of the harvested area in 2020 (FAO 2022). Besides being used for centuries as a source of sugar, the crop has an important role as a bioenergy feedstock. Renewable sources represent 45% of the Brazilian energy matrix, with sugarcane ethanol and bagasse corresponding to 39% (MME 2019).
In Brazil, sugarcane-derived ethanol use for transportation emits 82% less GHGs compared to petrol use (Jaiswal et al. 2017). In-field GHG emissions have progressively decreased in recent years with the phase-out of pre-harvest burning of sugarcane in Brazil, with the potential benefit of increased soil carbon sequestration from the decomposition of crop residues (Galdos et al. 2009, 2010). With the reduction of biomass burning and other sources of GHG, N2O emissions from fertiliser application, when converted to CO2-equivalent, have become increasingly important in the carbon footprint of sugarcane products.
Soil N2O emissions are highly variable due to factors such as climate conditions, soil properties, and management practices. The N2O emission factor (EF) default value for national inventories was recently updated in the guidelines of the Intergovernmental Panel on Climate Change (IPCC), e.g. EF of synthetic fertiliser (Tier 1) was 1.0% (0.3–3.0%) of N applied (IPCC 2006), and changed to disaggregated values of 0.5% (0.0–1.1%) in dry climate, and 1.6% (1.3–1.9%) in wet conditions (IPCC 2019). The average N2O-EF reported for sugarcane fields was 1.2% (1.0–1.5%) in a global study (Yang et al. 2021) and 0.7% (0.1–3.0%) in Brazil (Carvalho et al. 2021), which is fundamental to evaluating the environmental impact of ethanol.
Using an IPCC N2O-EF of 1.0%, Carvalho et al. (2021) demonstrated that the N2O emission can account for roughly half of the total GHG emission in bioethanol production. However, compared with IPCC values, the use of regional data (0.7%) reduced the total GHG emissions by 17, 18, and 21% when the fertilisers were ammonium nitrate (AN), urea, and ammonium sulphate (AS), respectively. A significant reduction in GHG emissions by choosing ethanol instead of petrol was evaluated considering N2O-EF of 1% (Cavalett et al. 2017), while N2O-EF as high as 5% could negate the carbon offsetting benefits of biofuels (Crutzen et al. 2007).
Site-specific crop and soil management conditions can lead to distinct results in total N2O emissions in sugarcane fields. Gonzaga et al. (2018) observed higher N2O-EF from N fertiliser by increasing straw levels from 0 to 15 Mg ha−1 (roughly 1 Mg ha−1 is equal to 4.0–4.5 kg N ha−1; Lourenco et al. 2018), but Vasconcelos et al. (2022) showed no effect of straw levels, and Pitombo et al. (2017) reported reduction in N2O-EF in soil covered with straw. Soares et al. (2016) reported a 95% reduction in N2O emission from urea adding nitrification inhibitors (Dicyandiamide-DCD and 3,4-dimethylpyrazole phosphate-DMPP), while Wang et al. (2016) showed a reduction of less than 36%. Combining application of nitrogen (N) fertiliser with stillage resulting from ethanol production (vinasse: 0.5–3.0 g N L−1) can lead to threefold increases in N2O emissions. In a study conducted by Lourenço et al. (2019), the N2O-EF from ammonium nitrate increased from 0.23% to 0.94% of N applied, and it reached 3% of N applied, when vinasse was applied at the same time as N fertilisers (Carmo et al. (2013).
Understanding how N2O fluxes are correlated with management and environmental conditions can help design strategies to mitigate emissions in sugarcane production. Soares et al. (2016) showed via a multiple linear regression (R2 = 0.47), N2O emissions correlating with the abundance of ammonia oxidising bacteria (AOB), precipitation, soil NH4+–N, NO3−–N, pH and CO2 emission due to N fertilisation in sugarcane in Brazil. Furthermore, Lourenço et al. (2018, 2022) expand the list of variables affecting N2O fluxes in sugarcane systems with straw and vinasse application, including factors such as bacterial genes linked to denitrification, ammonia oxidising archaea, fungal denitrifiers, air and soil temperature, and water-filled pore space.
Grouping individual studies can summarise the factors that may affect N2O emission in sugarcane fields. In a meta-analysis, Yang et al. (2021) showed higher cumulative N2O emissions when synthetic fertiliser was applied with organic amendments. On the other hand, no effect was observed in N2O-EF from N fertilisers due to the presence of straw (Abalos et al. 2022). Moreover, other management strategies that may influence N2O emissions from sugarcane fields, such as N sources, vinasse, and microbial activity, have yet to be investigated.
Therefore, management practices in sugarcane can affect the N2O emissions differently, and a better understanding of the factors influencing them can help in prediction and mitigation. To our knowledge, only one meta-analysis study has been published for N2O emissions in sugarcane (Yang et al. 2021), meaning our study with its rich datasets provides further analytical opportunities to understand such emissions, and fills a knowledge gap where little is known about the temporal influences on N2O fluxes. Thus, the aim of the present study was to identify the main factors that affect N2O emissions in sugarcane production, using a unique database containing daily N2O fluxes measured with a standard protocol in a variety of soil, climate, and management conditions.
Material and methods
Study sites and database
Flux measurements of N2O were obtained from 13 sugarcane trials conducted in the 2011–2017 period, at three experimental stations located in the main sugarcane-growing region in Brazil (Figure S1). The experiments involved management practices related to N fertilisation and organic amendments, such as various N fertiliser types and rates, vinasse application and post-harvest straw management (Table 1), which reflect both current management systems and mitigation alternatives (nitrification inhibitors and timing of vinasse application). All trials followed a similar experimental design, with each plot consisting of five rows of sugarcane spaced 1.5 m apart along a 10 m length, arranged into four blocks, each containing one or two chambers for gas sampling. The datasets were derived from a network of experiments conducted by researchers from the Agronomic Institute of Campinas (IAC) using the same protocol (Vargas 2013; Soares et al. 2015, 2016; Lourenço et al. 2018, 2019; Degaspari et al. 2020). The soils in the areas were classified as Red Latosol and Nitisol (Embrapa 2006).
Key variables for the soil, plant and atmosphere interface were measured in the trials, including GHG fluxes, climate data, stalk yields, crop residue (straw) rates, and soil chemical, physical, and biological properties (Table 2). Besides standard agronomic soil variables, the dataset includes abundances of the functional genes (archaeal and bacterial amoA, bacterial and fungal nirK, and bacterial nirS and nosZ), which encode proteins involved in nitrification and denitrification processes, and ribosomal RNA genes indicating total bacteria abundance (16S rRNA) and total fungi abundance (18S rRNA). The climate variables were obtained from weather stations located near the field plots (Ciiagro 2020).
GHG fluxes (CO2, CH4 and N2O) from all experiments were measured by cylindrical static chambers with 0.2 m in height and 0.3 m in diameter, inserted at a 0.05 m soil depth. Chambers were positioned in the inter-row (0.75 m from the sugarcane row) and partially in-row (0.10 m from the sugarcane row), to account for spatial differences in nitrogen fertilizer band application. Gases were sampled in the early morning three times per week during the first three months after fertiliser application, then biweekly. Three or four gas samples were collected in the 30 min following closure of the chambers, in either three (0–15–30 min) or four (0–10–20–30 min) measurements. After sampling, the gases were immediately stored in pre-evacuated Extainers® vials (Labco Limited, Ceredigion, United Kingdom) and analysed on a Shimadzu gas chromatograph (GC-2014). Although all three gases (CO2, CH4, and N2O) were analysed, this study focussed on N2O fluxes as a key component of the environmental footprint of sugarcane production.
The N2O flux was calculated by linear interpolation of the three or four sampling times (0–10–20–30 min), obtaining the angular coefficient. The cumulative N2O emission was calculated by linear interpolation between adjacent sampling dates. More details can be found in the studies used (Vargas 2013; Soares et al. 2015, 2016; Lourenço et al. 2018, 2019; Degaspari et al. 2020).
Descriptive, exploratory and inferential analyses
Overview
Descriptive, exploratory and inferential statistical analyses were undertaken to characterise the main factors that affect N2O emissions in sugarcane production. The original data from each trial was processed into a standard format for inclusion in the study database. Quality assessment included unit conversion, range tests, and visual inspection of homogeneity. The complete dataset was comprised of 49 quantitative and categorical variables from 13 trials encompassing a period of six years, with a total of close to 100,000 unique data points. When necessary, variables were transformed to deal with heavily skewed data and to promote robust analyses. For regression-type models, collinearity effects were catered for either by removal of strongly collinear data or within the model itself through some penalised term (e.g. a ridge term). Compositional data were handled simply by removing one class (e.g. remove clay say, from sand/silt/clay soil texture data). In the first instance, measurements for N2O and N2O-EFs were described, summarised, and visualised using simple boxplots, coupled with ANOVAs and related analyses.
Global and local temporal co-dependencies between N2O and rainfall and air temperature were explored via a series of cross-wavelet analyses. This analysis provided information on peak N2O fluxes given changes in the weather. Next, and similarly exploratory, a regression tree (RT) analysis was conducted to see how management, climate, and soil conditions influence N2O fluxes. Using a data subset directed by the RT analysis, correlation analyses were conducted to explore paired relationships and trends in the N2O processes and further highlight any collinear effects among the N2O predictor variables. Finally, an inferential multivariate analysis using linear regression was performed with N2O as the response, and whose coefficients were estimated using ordinary least squares (OLS) and assessed for significance from zero.
All analyses were conducted using R software, version 3.6.1 (R core team 2021), aside from the cross-wavelet analysis which was conducted using Python 3.6 with scripts from the PyCWT package (https://pypi.org/project/pycwt/). A detailed description of the statistical analyses can be found in Supplementary Material.
Differences in emission factors and emission intensity
The emission factor (EF) was calculated considering the cumulative N2O and N applied in the chambers for each treatment:
where EF is N2O emission factor (% of N applied); N2O treat (mg N m−2) and N2O control (mg N m−2) are the cumulative emissions of the fertilised and unfertilized chambers, respectively; and N applied is the amount of N (mg N m−2) added to the chamber as synthetic fertiliser and/or vinasse. The N2O emission intensity was calculated by dividing the cumulative N2O emissions in the season by the fresh weight yield of sugarcane stalks harvested.
The N2O-EFs were conditionally analysed according to the use of conventional fertilisers and for different mitigation treatments (nitrification inhibitor and timing of vinasse application). Firstly, this involved the simple presentation of conditional summary statistics (means, medians, standard deviations, IQRs, etc.) and boxplots. Secondly, formal analyses were conducted, using ANOVAs and Kruskal–Wallis (KW) rank sum tests (Vargha and Delaney 1998) in order to test whether EF variability was significantly different between conventional fertilisers and mitigation treatments. The ANOVAs and KW tests were supplemented by their respective post-hoc analysis (Tukey Honest Significant Differences—HSD) (Tukey, 1949) and the Dunn test (Dunn 1964) in order to determine which fertiliser or which mitigation had significantly different EF distributions (as an ANOVA or KW test only indicates at least one ‘category’ is different, but not which ‘category’). KW/Dunn tests represent robust alternatives to ANOVA/HSD tests, where the latter is resistant to outlying EFs (i.e., the different tests are analogous to the presentation of median/IQRs as well as means/SDs).
Results
Differences in emission factors and emission intensity
Daily N2O fluxes had a period of high emissions after fertiliser application, which was similar in all experiments and reached almost 150 mg N m−2 day−1 in trial C3 (Figure S2—top panel). The events of high emissions decrease until 100 DAF, showing low values afterwards with rare exceptions (Figure S2—middle panel). Some differences can be observed between trials with conditional boxplots, e.g., trial P2 had higher cumulative N2O emissions, which corresponded to a median of 710 mg N m−2, while J1 resulted in the lowest values, a median lower than 40 mg N m−2 (Figure S2—bottom panel).
Via the ANOVAs and associated tests, significant differences in N2O-EF between N sources were observed for each experiment group (Tables S1 and S2). In trials C1–C3—in which fertilisers were applied at 120 kg N ha−1 -UR and PSCU resulted in higher N2O-EF, with mean values of 1.0% and 1.2% of N applied, compared to UR containing nitrification inhibitors (DMPP and DCD) and calcium nitrate, which resulted in N2O-EF lower than 0.1%, as depicted in the conditional boxplots (Fig. 1). The UR treatment resulted in higher mean N2O-EF (1.0%) than CAN (0.6%) in P1–P3, with fertiliser rates ranging from 30 to 180 kg N ha−1. In P4-P5 and J1–J2—with fertiliser rates in the 50–150 kg N ha−1 range, the mean N2O-EFs were lower than 0.2% of N applied as AN and AS (Fig. 1), showing no statistically significant differences via the ANOVAs and associated tests (Table S1 and S2). In P6-P8 (with a uniform rate of 100 kg N ha−1), the application of AN with concentrated vinasse resulted in a mean N2O-EF of 1.3% of N applied (Fig. 1), where changing the time of vinasse application significantly decreased those emissions to 0.6% when vinasse was applied one month before AN, and to 0.3% when concentrated vinasse was applied one month after AN (see Tables S1 and S2).
Nitrous oxide emission factors (% of N fertiliser applied) according to N source in 13 sugarcane trials. AN: Ammonium Nitrate; AS: Ammonium Sulphate; CAN: Calcium Ammonium Nitrate; CN: Calcium Nitrate; PSCU: polymer sulphur -coated urea; UR: Urea; UR + DCD: Urea with Dicyandiamide; UR + DMPP: Urea with 3,4-Dimethylpyrazole Phosphate; V: Vinasse; CV: Concentrated vinasse
Grouping the same N source from different trials, the mean N2O-EFs ranged from 0.03 to 1.17% of N applied (Table 3). The highest N2O-EFs were UR and PSCU, with mean values of 0.98% and 1.17%, respectively. Mean EFs for CAN (0.57%) and AN (0.50%) were higher than CN, AS, and UR + Nitrification Inhibitors (NIs; DCD and DMPP), with N2O-EFs of 0.1% of N applied or lower (Table 3). A weighted average of EFs considering the number of treatments in each fertiliser type, excluding the mitigation treatments (UR + DCD and UR + DMPP) and the coated urea treatment (PSCU), amounts to a mean EF of 0.6 (± 0.03)%. The intensity of N2O emission ranged from 2 to 150 g N2O per Mg of sugarcane stalk yield (Figure S3). The N2O intensity results were similar to those with N2O-EF, with higher values with UR and AN combined with concentrated vinasse. The N rate increased N2O intensity for UR, AN, AS, and CAN (Figure S3).
Cross-wavelet analysis on emissions time series data
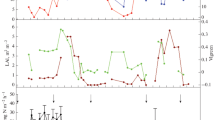
Cross-wavelet analyses were carried out between N2O fluxes and two variables: rainfall and air temperature (Fig. 2 and Figure S4, respectively). The examples presented refer to the 2012–2014 trial number 1, with application of UR at the rate of 120 kg N ha−1. Examples were chosen as representatives of the general pattern observed in most of the experiments. Cross wavelet power, which is proportional to the covariance between rainfall and N2O flux (Fig. 2), is shown in the bottom panel in a logarithmic colour scale; in this panel, the arc-shaped shading denotes areas with low confidence due to edge effects near time series start and end. The black contours enclose regions where the cross-wavelet power is significantly different from a red noise background. Here a red noise is defined as a signal with a spectral energy density proportional to the reciprocal of the frequency squared. Finally, the arrows’ angle—in clockwise direction—denote the phase difference between peaks in the time series; upwards arrows (zero degrees) indicate perfectly aligned peaks, while right pointing arrows (90 degrees) indicate that rainfall leads the N2O flux, i.e., peaks in the rainfall series precede fluxes with lead times depending on the time scale on the vertical axis. Lead time τ is calculated as τ = (θ/360)P, where θ is the arrow angle, in degrees, and P is the cross-wavelet period. For example, the first event with significant cross-wavelet power in Fig. 2 occurred in April 2012, between time scale P of 2 to 64 days. The phase arrows point mostly upwards, indicating that rainfall peaks and N2O fluxes were mostly in sync in the monthly scale. The same patterns of upward arrows were observed for the experiment in 2014, up to the time scale of 64 days. However, short-lived peaks of N2O were observed to be delayed by 2–4 days in relation to rainfall events in April 2012. During that period, the cross-wavelet power is highest (colour scale) around the time scale of 8 days, which is consistent with the N2O’s peak width; the arrows in this region of the cross-wavelet panel are tilted around 90°–120°, which would result in N2O peaks with delays of 2–3 days after the rainfall. Lags were not observed during the periods in November 2012 or January 2014. However, according to the data records, the peak of 119.3 mg N m−2 day−1 on January 2nd, 2014 was preceded by a 39 mm in rainfall on December 29th, 2013; the subsequent peak of 98.1 mg N m−2 day−1 on January 17th, 2014 occurred after a rainfall event of 37.3 mm on January 15th, 2014. These lags are not evident in the cross-wavelet panel due to the limitations in the temporal resolution of chamber measurements, which took place every 3 days.
Cross-wavelet analysis between rainfall (a) and N2O flux (b) measured at trial C1 from 2012 to 2014. Cross-wavelet power (c) on log2 scale with units proportional to the covariance between the two signals. The area strongly influenced by the signals' edges is not considered and marked with a hatched pattern. The black contours enclose regions where the cross-wavelet power is statistically significant against a red noise background. Arrow angles represent the phase between the signals (clockwise reference). Fertiliser application dates marked with vertical dashed lines
Peak N2O fluxes were significantly correlated with daily mean temperature at various time scales, with no clear pattern of temporal delays (Figure S4). This result suggests that N2O fluxes might be triggered after a threshold in air temperature, depending on N availability in soil, rainfall events and soil moisture conditions.
Classification and regression tree analysis for daily emissions
The RT analysis indicated that high N2O fluxes were distinguished from low values according to management, climate, and soil conditions (Fig. 3). The driver hierarchy in the RT model included DAF, fertiliser -N rate, N source, Tmin, pH, CO2, Vinasse, and Tmax. The first partitioning was due to DAF, in which low N2O-N fluxes are expected to occur when DAF > 47 days, independent of other conditions. The model predicted N2O-N emission of 0.41 mg N m−2 day−1 in DAF > 47 (Fig. 3). Low N2O emissions (< 1.0 mg N m−2 day−1) were also observed with DAF < 47, without N addition or with the N sources AN, AS, CN, or UR + inhibitors, and CO2 flux < 6.3 g C m−2. On the other hand, the highest N2O-N fluxes estimated were 37 mg N m−2 day−1, related to N sources CAN, PSC, or UR, in DAF < 29 days, N rate > 150 kg N ha−1 and Tmin > 18 °C (Fig. 3). High fluxes were also correlated following: DAF < 47 days CO2 > 6.3 g C m−2, and the N source AN with CV; DAF < 47, N source CAN, PSC, or UR, N rate < 150 kg N ha−1, Tmin > 18 °C, and pH 5.4–6.1; and DAF < 47, N rate < 150 kg N ha−1, Tmin > 18 °C, pH > 6.1, CO2 > 4.5 g C m−2, Tmax < 32 °C, with UR or PSC (Fig. 3).
Regression tree (RT) relationship of N2O fluxes (mg N m−2 day−1) with management, climate, and soil conditions in the 13 sugarcane trials. The colour code in the figure ranges from blue (low emissions) to red (high emissions). DAF: days after fertiliser application (days); Fert_Rate: rate of N fertiliser (kg N ha−1); Tmin/Tmax: minimum/maximum temperature (°C); CO2 fluxes (g C m−2 day−1); OM: soil organic matter (mg kg−1); Prec: precipitation (mm/day); BD: Bulk density; P: phosphorus content in soil (mg P dm−3); n = number of observations
Correlation and regression analysis with a reduced database
Cumulative N2O emission, in 46 days resulted in positive and negative correlations with key soil, climate and management variables (Fig. 4). The highest correlation was found between N2O and N fertiliser rate, with a coefficient r = 0.69 (p < 0.001). Significant positive correlations with N2O were found for 18S rRNA gene (r = 0.66, p < 0.001), NH4+ (r = 0.58, p < 0.001), NO3− (r = 0.57, p < 0.001), nirk fungi gene (r = 0.35, p < 0.01), silt content (r = 0.26, p < 0.001), soil P (r = 0.22, p < 0.001), and total precipitation in the period (r = 0.20, p < 0.01). Significant negative correlations were observed with N2O and archaeal amoA (r = − 0.37, p < 0.01), soil organic matter (r = − 0.34, p < 0.001), bulk density (r = − 0.23, p < 0.001), CEC (r = − 0.19, p < 0.01), V (r = − 0.16, p < 0.01), and Ca (− 0.14, p = 0.02). No significant correlations were identified for other variables, including average maximum and minimum air temperature, despite apparent trends for higher emissions with higher temperature values (Figure S5).
According to multiple linear regression fits, the full set of variables including nitrogen fertiliser rate, straw rate, total precipitation, average maximum and minimum air temperature, and soil macro- and micro-nutrients, texture, CEC and WFPS explained ~ 65% of the cumulative N2O emission, suggesting that unmeasured biotic or abiotic factors explained the remaining 35% of the variation (Table 4). When the set of variables was reduced to include only parameters easily obtained by farmers such as fertiliser rate, the amount of straw left on the field after harvest, rainfall, and temperature on site, the regression still explained over 50% of the variation.
Discussion
Fertiliser type significantly influenced emission factors and GHG intensity
Mean emission factors ranged from 0.03 to 1.17% of N applied over the thirteen experiment-years. These results combined the main management practices applied to sugarcane in Brazil and are in line with individual studies published (Carmo et al. 2013; Paredes et al. 2014; Soares et al. 2015; Siqueira Neto et al. 2016; Silva et al. 2017; Pitombo et al. 2017; Gonzaga et al. 2018; Borges et al. 2019; Lourenço et al. 2019; Degaspari et al. 2020; Cabral et al. 2020; Vasconcelos et al. 2022). In the present study, the mean values for N2O-EF, excluding nitrification inhibitor treatments, was 0.6%, which is lower than mean values previously reported, such as 0.7% for sugarcane Brazil (Carvalho et al. 2021), 1.2% for global sugarcane (Yang et al. 2021), and the default value of 1.6% from IPCC Tier 1 for regions with annual rainfall > 1000 mm (IPCC 2019). The N2O-EF depends on site-specific conditions, such as management, soil, and climate. In this way, a more detailed prediction as models (Tier 3) can better estimate N2O-EF (IPCC 2019) and the sustainability of the agricultural product.
Summarising N2O-EF by N sources, different values were found; using ammonium-based fertilisers, the mean N2O-EF was 0.5–0.6% (AN and CAN), while using UR-based sources it was 1% of N applied, but other N sources had N2O-EF lower than 0.1%. The range of N2O-EF occurred due to different management, e.g., the highest mean value for a treatment (3.15%) was observed when AN at 100 kg N ha−1 was combined with concentrated vinasse. Other high values of N2O-EFs included UR or CAN applied at a high N rate (180 kg ha−1) in soil covered with straw. In a meta-analysis study of N2O emission in sugarcane, Yang et al. (2021) observed higher emissions when combining synthetic fertiliser with organic amendments (N2O-EF: 2.47%) and increasing N rate application. On the other hand, low N2O-EFs (< 0.10%) were found in the present study, with CN, AN, and AS, as well as in the mitigation options: nitrification inhibitors addition in UR; and anticipated/postponed vinasse combined with AN. Therefore, the N management in sugarcane can show low N2O-EF options, which can be a strategy to mitigate GHG emissions (Carvalho et al. 2021). N2O emission intensity, represented as the cumulative N2O emissions in a growing season normalised by sugarcane yield, followed similar patterns to emission factors, with UR intensity generally higher than other fertiliser types. Fertiliser rates influenced N2O intensity, indicating that the increase in GHG emissions from excessive N application is not compensated by proportional increases in yield, as described by Takeda et al (2021) in a study monitoring emissions in a sugarcane plantation in Australia. Additionally, the emissions intensity of concentrated vinasse applied concomitantly with N fertiliser was significantly higher than emission intensity when vinasse was applied either 30 days before or 30 days after fertiliser, demonstrating the synergistic effect of combining organic amendments and mineral fertilisers on N2O emissions. When vinasse, an organic amendment with high moisture content, is applied along with fertiliser, a combination of increased soil moisture and corresponding increase in water filled pore space; and more nitrogen and labile carbon available for the soil microbial community can lead to increased soil N2O emissions, without a corresponding increase in sugarcane yields.
N2O emissions were related to days after fertiliser application
When compared to the other variables studied, N management had the greatest impact on daily N2O flux. The high N2O fluxes occurred close to the time of N application, DAF < 47 days (Fig. 2); in addition, the N rate showed the highest correlation with cumulative N2O emission (Fig. 3) and was included in the OLS regression (Table 4). Increasing the N rate reduces nitrogen use efficiency in sugarcane (Chalco Vera et al. 2022; Sanches and Otto 2022), and increases N2O emissions, potentially priming organic N mineralization from soil (Degaspari et al. 2020; Takeda et al. 2022). In a study in Australia, Takeda et al. (2021) report that increasing fertiliser application beyond the recommended rate of 200 kg N ha−1 led to doubling the amount of N2O emitted per kg sugar yield—indicating an exponential effect.
The amount of straw left on the field did not have significant correlation with cumulative N2O emissions and was only significant in the linear regression with the reduced set of variables. The effect of straw on N2O emissions has been shown to have contrasting results as positive (Gonzaga et al. 2018) and negative effects (Pitombo et al. 2017). In a recent meta-analysis study, Abalos et al. (2022) reported an increase in N2O emissions from fresh crop residues (cover crops, grasslands and vegetables) but not from partially decomposed residues, with sugarcane postharvest straw showing no effect. Sugarcane straw can display diverse decomposition stages linked to accumulated residues from previous harvests, affecting soil microbial community and N2O emissions (Galdos et al 2010; Pitombo et al. 2017; Gonzaga et al. 2018).
Environmental controls of N2O emissions
In the period close to fertiliser application, our analysis showed the temporal relationship (wavelet) impact of rainfall and air temperature on N2O fluxes. Zeri et al. (2020) showed that air temperature precedes the N2O peak by 10–20 days for biofuel crops (maize, miscanthus, switchgrass, and prairie). Here we found that the time lag between rainfall and N2O emission in sugarcane fields was 2 to 4 days on average during the first experiment in 2012, but not evident in the remaining measurements. According to Fig. 2b, N2O emissions were observed weeks after fertiliser application dates. The trigger is most likely soil moisture conditions (WFPS) reaching a threshold that enhances N2O production. The relationship between WFPS of 70% as a trigger to N2O emissions was reported by Metay et al. (2011), using simulations, and by Liang et al. (2018) over grazed pastures, which also associated the emissions to moderate soil temperature. The delays observed in April 2012 are most likely related to specific soil conditions at the time, or rainfall intensity, resulting in different soil wetting processes.
Soil moisture and temperature are the critical factors affecting microbial activity and diversity (Paul and Clark 1989). In dry conditions, soil moisture is more important than temperature, but when moisture is not limited, temperature is the major factor (Paul and Clark 1989). In general, higher production of N2O emissions in soil is expected to occur in WFPS between 50 and 75%, which is a more favourable condition for both nitrification and denitrification processes (Del Grosso et al. 2002; Liu et al. 2007). Increasing the temperature increases the rates of nitrification (Di and Cameron 2004) and denitrification processes (Braker et al. 2010), increasing N2O emissions. Chalco Vera et al. (2020) reported high N2O emissions increasing soil temperature (> 19 °C) and soil moisture (> 29.2%). However, Vargas et al. (2019) found that N2O emissions were higher in soil with temperature at 20 °C than at 30 °C. The study was conducted in controlled conditions, where the straw with a high C/N ratio probably causes a higher N immobilisation at higher temperatures. In the present study, over thirteen experiment-years of observation in the field, the N was applied in a band in soil (1.5 m row spacing), which elevates the soil N concentration and probably reduces the immobilisation effect compared with the broadcast-basis application of Vargas et al. (2019).
The delays found between N2O fluxes with rainfall and air temperature in cross wavelet analysis help to explain the low correlation (Pearson, r < 0.1) found between fluxes and both precipitation and air temperature in the linear regression analysis. Two sinusoidal curves which are completely in phase would result in a perfect correlation (r = 1) since peaks and valleys are aligned. Conversely, a phase difference, or delay, of 180 rad degrees would result in an inverse correlation (r = − 1). However, in-between delays such as 90 degrees result in misaligned peaks and valleys, and consequent zero correlation. The N2O fluxes are a result of microbial processes in the soil, which are dependent on substrate availability (N), in addition to ideal conditions of soil temperature and moisture. Rainfall events were frequent during the occurrence of the N2O peaks, most likely creating ideal conditions of soil moisture for microbial activity and N2O fluxes. It should be noted that soil moisture and soil temperature were not available in all GHG sampling points in the trials, and rainfall and air temperature were used as proxies to represent thermal and moisture conditions in the soil.revious results on cross-wavelet analysis of N2O fluxes found no significant differences in delays when using air temperature or soil temperature (Zeri et al. 2020).
Fungi abundance had a positive correlation with N2O emissions
Several factors had an impact on N2O fluxes, with strong positive correlations with fertiliser application rates, soil NH4+ and NO3− content, and 18S rRNA genes, weak positive correlations with nirk fungi genes; and weak negative correlations with genes archaeal amoA (AOA) and soil organic matter content. The addition of organic C via vinasse and straw can result in an increase in microbial activity and in N2O emissions, where it could have more impact in soils with low OM (Lourenço et al. 2018). This effect can also explain the correlation of N2O with fungal activity (18S rRNA and nirk fungi genes), where the organic material supports favourable conditions for fungi growth, such as high moisture and organic C, leading to increases in N2O emissions (Lourenço et al. 2022). However, Yang et al. (2021) observed higher N2O emissions in soil with SOC > 20 g C dm−3 than with lower content; probably the organic C added in our study had a higher impact where soil C content was lower (11–16 g C dm−3), increasing N2O emissions. It is interesting that the relationship of N2O with bacterial amoA (AOB) was not significant, contrary to previous studies (Soares et al. 2016; Lourenço et al. 2018). The amoA gene codifies the nitrification process, which was the main process in individual studies, such as Soares et al. (2016) using UR in soil with no straw. Our results indicate that besides taking into account climate, management, and soil chemistry variables, soil microbiology should also be considered when monitoring N2O fluxes in sugarcane fields, a first step towards reducing emissions and reducing the carbon footprint of sugar and ethanol.
Policy perspectives for GHG mitigation
Excessive input of reactive nitrogen (Nr) leads to high N losses posing significant threats to water, air, soil, biodiversity as well as GHG balance via direct and indirect emission of N2O (Galloway et al. 2004; Butterbach-Bahl et al. 2011). Aiming to meet the commitments of the Paris Agreement under the UNFCCC, the Brazilian government launched Law 13.576/2017, named “Renovabio”, which encourages the expansion of biofuel production to contribute to environmental preservation by reducing the emissions of GHG and other polluting gases (e.g., nitrogen oxides, particulate matter, etc.), in addition to promoting fuel security and economic and social development. The policy has been successfully adopted, as 90% of total ethanol plants in Brazil were certified in 2021 (Rossetto et al. 2022), motivated by the GHG reduction and economic benefits (Cantarella et al 2023).
Although sugarcane is considered the most suitable crop for bioenergy production worldwide (Miller 2010; Moraes and Zilberman 2014; Otto et al. 2022), its sustainability has been questioned due to its environmental and social impacts (Martinelli and Filoso 2008; Robinson et al. 2011; Immerzeel et al. 2014). Ways of reducing many negative impacts were addressed in the Renovabio legislation as bioenergy producers must comply with rules to benefit from decarbonization credits. For instance, bioenergy crops must not be grown in areas that have been deforested, even legally, after December 2017, crops must come from zoning areas allowed for specific feedstocks, and producers must abide by the Forest Code (Brasil 2017). In fact, sugarcane expansion occurred mostly in areas of pasture and cropland, where forest corresponded to less than 1% (Adami et al. 2012; Cherubin et al. 2021).
Problems mainly arise from the relatively low N-fertiliser recovery by sugarcane and the consequent high losses to the soil system (Chapman et al. 1994; Franco et al. 2011). Historically, Brazil has had a comparatively low input of N-fertiliser in sugarcane production (Baldani et al. 2002; de Matos Nogueira et al. 2005; Miller 2010). However, the expansion of sugarcane over the last decades increased the consumption of synthetic N-fertilisers. This is a complex situation, as fertiliser consumption is a critical indicator that determines the sustainability of nitrogen management in the country (Cunha-Zeri et al. 2022).
The present study identifies the main factors that affect N2O emissions in sugarcane production. An underlying factor is that as more fertiliser is used in sugarcane plantations, the risk of nitrogen being lost to the surrounding environment and causing pollution increases (Martinelli and Filoso 2008; Sutton et al. 2011). Therefore, monitoring in-field N2O fluxes is essential to ensure that the sustainability of sugarcane ethanol produced in Brazil remains favourable. Using IPCC tier 1 default values, Carvalho et al. (2021) estimated the cradle to wheel C footprint of ethanol and reported that ethanol reduced GHG emissions in 73% when replacing petrol; the avoided emissions could be increased by an additional 21% by adding nitrification inhibitors to N fertilisers used to produce sugarcane. Other policy responses should include strategies and investments in new technologies for sustainable agriculture, as well as a circular economy for nitrogen aimed at optimising the efficiency of resource use without waste and pollution (Sutton et al. 2019).
Conclusions
The present study identifies important factors controlling N2O emissions in sugarcane fields in Southeastern Brazil. The N management had a high impact, where the high N2O fluxes occurred in the first 46 days after fertiliser application. There were also significant positive correlations between cumulative N2O and N rate, soil NH4+ and NO3−, and the genes 18S and fungal nirk; and negative correlations with organic matter and genes archaeal amoA. The mean N2O-EFs ranged from 0.03 to 1.17% of the N applied, with high emissions occurring with urea (UR), increasing N rate, and applying ammonium nitrate (AN) combined with vinasse, and low N2O-EF occurring with ammonium sulphate, AN, calcium nitrate, and mitigation alternatives (nitrification inhibitors and timing of vinasse application). The rainfall and air temperature had a high influence on the emissions, preceding the N2O fluxes by 2 to 4 days in some experiments and being generally well correlated overall. Understanding the factors influencing N2O emissions in sugarcane production, including fertiliser and organic amendment type, timing and rate, crop residue management, climate and soil variables can help farmers, extensionists, researchers and policy makers in the development and implementation of climate change mitigation strategies.
References
Abalos D, Rittl TF, Recous S et al (2022) Predicting field N2O emissions from crop residues based on their biochemical composition: a meta-analytical approach. Sci Total Environ 812:152532. https://doi.org/10.1016/j.scitotenv.2021.152532
Adami M, Rudorff BFT, Freitas RM et al (2012) Remote sensing time series to evaluate direct land use change of recent expanded sugarcane crop in Brazil. Sustainability 4:574–585. https://doi.org/10.3390/su4040574
Baldani JI, Reis VM, Baldani VLD, Döbereiner J (2002) Review: a brief story of nitrogen fixation in sugarcane—reasons for success in Brazil. Funct Plant Biol 29:417–423. https://doi.org/10.1071/pp01083
Borges CD, Carvalho JLN, Kölln OT et al (2019) Can alternative N-fertilization methods influence GHG emissions and biomass production in sugarcane fields? Biomass Bioenergy 120:21–27. https://doi.org/10.1016/j.biombioe.2018.10.017
Braker G, Schwarz J, Conrad R (2010) Influence of temperature on the composition and activity of denitrifying soil communities: temperature effects on soil denitrifier communities. FEMS Microbiol Ecol. https://doi.org/10.1111/j.1574-6941.2010.00884.x
Brasil (2017). Brasil, “Lei No. 13576, de 26 de Dezembro de 2017—Dispõe Sobre a Política Nacional de Biocombustíveis (RenovaBio) e dá Outras Providências. 2017. Available online: https://www.planalto.gov.br/ccivil_03/_ato2015-2018/2017/lei/l13576.htm
Breiman L, Friedman JH, Olshen RA, Stone CJ (2017) Classification and regression trees. Routledge, New York
Butterbach-Bahl K, Nemitz E, Zaehle S et al (2011) Nitrogen as a threat to the European greenhouse balance. Eur Nitrogen Assess Sources Eff Policy Perspect 19:434–462
Butterbach-Bahl K, Baggs EM, Dannenmann M et al (2013) Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos Trans R Soc Lond B Biol Sci 368:20130122. https://doi.org/10.1098/rstb.2013.0122
Cabral OMR, Freitas HC, Cuadra SV et al (2020) The sustainability of a sugarcane plantation in Brazil assessed by the eddy covariance fluxes of greenhouse gases. Agric for Meteorol 282–283:107864. https://doi.org/10.1016/j.agrformet.2019.107864
Cantarella H, Silva JFL, Nogueira LAH et al (2023) Biofuel technologies: lessons learned and pathways to decarbonization. GCB Bionergy. https://doi.org/10.1111/gcbb.13091
Carmo JB, do, Filoso S, Zotelli LC, et al (2013) Infield greenhouse gas emissions from sugarcane soils in Brazil: effects from synthetic and organic fertilizer application and crop trash accumulation. GCB Bioenergy 5:267–280. https://doi.org/10.1111/j.1757-1707.2012.01199.x
Carvalho JLN, Oliveira BG, Cantarella H et al (2021) Implications of regional N2O-N emission factors on sugarcane ethanol emissions and granted decarbonization certificates. Renew Sustain Energy Rev 149:111423. https://doi.org/10.1016/j.rser.2021.111423
Cavalett O, Chagas MF, Junqueira TL et al (2017) Environmental impacts of technology learning curve for cellulosic ethanol in Brazil. Ind Crops Prod 106:31–39. https://doi.org/10.1016/j.indcrop.2016.11.025
Chalco Vera J, Curti R, Acreche MM (2020) Integrating critical values of soil drivers for mitigating GHGs: an assessment in a sugarcane cropping system. Sci Tot Environ 704:135420. https://doi.org/10.1016/j.scitotenv.2019.135420
Chalco Vera J, Portocarrero R, Piñeiro G, Acreche MM (2022) Increases in nitrogen use efficiency decrease nitrous oxide emissions but can penalize yield in sugarcane. Nutr Cycl Agroecosyst 122:41–57. https://doi.org/10.1007/s10705-021-10180-3
Chapman LS, Haysom MBC, Saffigna PG (1994) The recovery of 15N from labelled urea fertilizer in crop components of sugarcane and in soil profiles. Aust J Agric Res 45:1577–1585. https://doi.org/10.1071/ar9941577
Cherubin MR, Carvalho JLN, Cerri CEP et al (2021) Land use and management effects on sustainable sugarcane-derived bioenergy. Land 10:72. https://doi.org/10.3390/land10010072
Ciiagro (2020) Portal Agrometeorológico e Hídrológico do Estado de São Paulo. http://www.ciiagro.org.br/. Accessed 8 Sept 2020
Crutzen PJ, Mosier AR, Smith KA, Winiwarter W (2007) N2O release from agro-biofuel production negates global warming reduction by replacing fossil fuels. Atmos Chem Phys Discuss 7:11191–11205
Cunha-Zeri G, Guidolini JF, Branco EA, Ometto JP (2022) How sustainable is the nitrogen management in Brazil? A sustainability assessment using the entropy weight method. J Environ Manag 316:115330. https://doi.org/10.1016/j.jenvman.2022.115330
de Matos NE, Olivares FL, Japiassu JC et al (2005) Characterization of glutamine synthetase genes in sugarcane genotypes with different rates of biological nitrogen fixation. Plant Sci 169:819–832. https://doi.org/10.1016/j.plantsci.2005.05.031
Degaspari IAM, Soares JR, Montezano ZF et al (2020) Nitrogen sources and application rates affect emissions of N2O and NH3 in sugarcane. Nutr Cycl Agroecosyst. https://doi.org/10.1007/s10705-019-10045-w
Del Grosso S, Ojima D, Parton W et al (2002) Simulated effects of dryland cropping intensification on soil organic matter and greenhouse gas exchanges using the DAYCENT ecosystem model. Environ Pollut 116(Supplement 1):S75–S83. https://doi.org/10.1016/S0269-7491(01)00260-3
Di HJ, Cameron KC (2004) Effects of temperature and application rate of a nitrification inhibitor, dicyandiamide (DCD), on nitrification rate and microbial biomass in a grazed pasture soil. Soil Res 42:927. https://doi.org/10.1071/SR04050
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:241–252. https://doi.org/10.1080/00401706.1964.10490181
Embrapa (2006) Sistema Brasileiro de Classificação de Solos. Embrapa Solos, Rio de Janeiro (in portuguese)
FAO (2022) FAOSTAT-data. In: FAOSTAT. http://www.fao.org/faostat/en/#data/EL. Accessed 10 Aug 2022
Franco HCJ, Otto R, Faroni CE et al (2011) Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crops Res 121:29–41. https://doi.org/10.1016/j.fcr.2010.11.011
Furon AC, Wagner-Riddle C, Smith CR, Warland JS (2008) Wavelet analysis of wintertime and spring thaw CO2 and N2O fluxes from agricultural fields. Agric for Meteorol 148:1305–1317. https://doi.org/10.1016/j.agrformet.2008.03.006
Galdos MV, Cerri CC, Cerri CEP (2009) Soil carbon stocks under burned and unburned sugarcane in Brazil. Geoderma 153:347–352. https://doi.org/10.1016/j.geoderma.2009.08.025
Galdos MV, Cerri CC, Cerri CEP et al (2010) Simulation of sugarcane residue decomposition and aboveground growth. Plant Soil 326:243–259. https://doi.org/10.1007/s11104-009-0004-3
Galloway JN, Dentener FJ, Capone DG et al (2004) Nitrogen cycles: past, present, and future. Biogeochemistry 70:153–226. https://doi.org/10.1007/s10533-004-0370-0
Gonzaga LC, Carvalho JLN, de Oliveira BG et al (2018) Crop residue removal and nitrification inhibitor application as strategies to mitigate N2O emissions in sugarcane fields. Biomass Bioenergy 119:206–216. https://doi.org/10.1016/j.biombioe.2018.09.015
Grinsted A, Moore JC, Jevrejeva S (2004) Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process Geophys 11:561–566. https://doi.org/10.5194/npg-11-561-2004
Immerzeel DJ, Verweij PA, van der Hilst F, Faaij APC (2014) Biodiversity impacts of bioenergy crop production: a state-of-the-art review. GCB Bioenergy 6:183–209. https://doi.org/10.1111/gcbb.12067
IPCC (2006) Guidelines for national greenhouse gas inventories. IGES, Japan
IPCC (2019) 2019 Refinement to the 2006 IPCC guidelines for national greenhouse gas inventories. Institute for Global Environmental Strategies Hayama, Japan
IPCC (2021) Climate change 2021: the physical science basis. Contribution of Working Group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press
Jaiswal D, De Souza AP, Larsen S et al (2017) Brazilian sugarcane ethanol as an expandable green alternative to crude oil use. Nat Clim Change Adv. https://doi.org/10.1038/nclimate3410
Liang LL, Campbell DI, Wall AM et al (2018) Nitrous oxide fluxes determined by continuous eddy covariance measurements from intensively grazed pastures: temporal patterns and environmental controls. Agric Ecosyst Environ 268:171–180. https://doi.org/10.1016/j.agee.2018.09.010
Liu XJ, Mosier AR, Halvorson AD et al (2007) Dinitrogen and N2O emissions in arable soils: effect of tillage, N source and soil moisture. Soil Biol Biochem 39:2362–2370. https://doi.org/10.1016/j.soilbio.2007.04.008
Lourenço KS, Dimitrov MR, Pijl A et al (2018) Dominance of bacterial ammonium oxidizers and fungal denitrifiers in the complex nitrogen cycle pathways related to nitrous oxide emission. GCB Bioenergy. https://doi.org/10.1111/gcbb.12519
Lourenço KS, Rossetto R, Vitti AC et al (2019) Strategies to mitigate the nitrous oxide emissions from nitrogen fertilizer applied with organic fertilizer in sugarcane. Sci Total Environ 650:1476–1486. https://doi.org/10.1016/j.scitotenv.2018.09.037
Lourenço KS, de Costa OY, A, Cantarella H, Kuramae EE, (2022) Ammonia-oxidizing bacteria and fungal denitrifier diversity are associated with N2O production in tropical soils. Soil Biol Biochem 166:108563. https://doi.org/10.1016/j.soilbio.2022.108563
Martinelli LA, Filoso S (2008) Expansion of sugarcane ethanol production in Brazil: environmental and social challenges. Ecol Appl Publ Ecol Soc Am 18:885–898. https://doi.org/10.1890/07-1813.1
Metay A, Chapuis-Lardy L, Findeling A et al (2011) Simulating N2O fluxes from a Brazilian cropped soil with contrasted tillage practices. Agric Ecosyst Environ 140:255–263. https://doi.org/10.1016/j.agee.2010.12.012
Milborrow S (2022) rpart.plot: Plot “rpart” models: an enhanced version of “plot.rpart”. R package version 3.0.8. https://CRAN.R-project.org/package=rpart.plot
Miller SA (2010) Minimizing land use and nitrogen intensity of bioenergy. Environ Sci Technol 44:3932–3939. https://doi.org/10.1021/es902405a
MME (2019) Ministry of Mines and Energy (MME). Resenha Energética Brasileira - edição 2019 (in portuguese)
Moraes MAFD, Zilberman D (2014) Production of ethanol from sugarcane in Brazil. Springer, Cham
Otto R, Ferraz-Almeida R, Sanches GM et al (2022) Nitrogen fertilizer consumption and nitrous oxide emissions associated with ethanol production—a national-scale comparison between Brazilian sugarcane and corn in the United States. J Clean Prod 350:131482. https://doi.org/10.1016/j.jclepro.2022.131482
Paredes DS, da Lessa AC, R, de Sant’Anna SAC, et al (2014) Nitrous oxide emission and ammonia volatilization induced by vinasse and N fertilizer application in a sugarcane crop at Rio de Janeiro, Brazil. Nutr Cycl Agroecosyst 98:41–55. https://doi.org/10.1007/s10705-013-9594-5
Paul EA, Clark FE (eds) (1989) Soil microbiology and biochemistry. Academic Press, San Diego
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team (2019) Nlme: linear and nonlinear mixed effects models. R Package Version 3.1-141. https://CRAN.R-project.org/package=nlme
Pitombo LM, Cantarella H, Packer APC et al (2017) Straw preservation reduced total N2O emissions from a sugarcane field. Soil Use Manag 33:583–594. https://doi.org/10.1111/sum.12384
R core team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austria. https://www.R-project.org/
Robinson N, Brackin R, Vinall K et al (2011) Nitrate paradigm does not hold up for sugarcane. PLoS ONE 6:e19045. https://doi.org/10.1371/journal.pone.0019045
Rossetto R, Ramos NP, de Matos Pires RC et al (2022) Sustainability in sugarcane supply chain in Brazil: issues and way forward. Sugar Tech 24:941–966. https://doi.org/10.1007/s12355-022-01170-y
Rushworth A, Wilkins (2021) inspectdf: Inspection, comparison and visualisation of data frames. R package version 0.0.11 https://CRAN.R-project.org/package=inspectdf
Sanches GM, Otto R (2022) A novel approach for determining nitrogen requirement based on a new agronomic principle—sugarcane as a crop model. Plant Soil 472:29–43. https://doi.org/10.1007/s11104-021-05263-7
Silva JF, Moreira de Carvalho A, Rein TA et al (2017) Nitrous oxide emissions from sugarcane fields in the Brazilian Cerrado. Agric Ecosyst Environ 246:55–65. https://doi.org/10.1016/j.agee.2017.05.019
Siqueira Neto M, Galdos MV, Feigl BJ et al (2016) Direct N2O emission factors for synthetic N-fertilizer and organic residues applied on sugarcane for bioethanol production in Central-Southern Brazil. GCB Bioenergy 8:269–280. https://doi.org/10.1111/gcbb.12251
Soares JR, Cantarella H, Vargas VP et al (2015) Enhanced-efficiency fertilizers in nitrous oxide emissions from urea applied to sugarcane. J Environ Qual 44:423. https://doi.org/10.2134/jeq2014.02.0096
Soares JR, Cassman NA, Kielak AM et al (2016) Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci Rep. https://doi.org/10.1038/srep30349
Sutton MA, Oenema O, Erisman JW et al (2011) Too much of a good thing. Nature 472:159–161. https://doi.org/10.1038/472159a
Sutton M, Haghuram M, Adhya TK (2019) The nitrogen fix: from nitrogen cycle pollution to nitrogen circular economy. In: Frontiers 2018/19: emerging issues of environmental concern. United Nations Environment Programme, Nairobi, Kenya
Takeda N, Friedl J, Rowlings D et al (2021) Exponential response of nitrogen oxide (N2O) emissions to increasing nitrogen fertiliser rates in a tropical sugarcane cropping system. Agric Ecosyst Environ 313:107376. https://doi.org/10.1016/j.agee.2021.107376
Takeda N, Friedl J, Kirkby R et al (2022) Interaction between soil and fertiliser nitrogen drives plant nitrogen uptake and nitrous oxide (N2O) emissions in tropical sugarcane systems. Plant Soil. https://doi.org/10.1007/s11104-022-05458-6
Therneau T, Atkinson B, Ripley B (2022) rpart: Recursive partitioning and regression trees. R package version 4.1.16. https://CRAN.R-project.org/package=rpart
Tian H, Xu R, Canadell JG et al (2020) A comprehensive quantification of global nitrous oxide sources and sinks. Nature 586:248–256. https://doi.org/10.1038/s41586-020-2780-0
Torrence C, Compo GP (1998) A practical guide to wavelet analysis. Bull Am Meteorol Soc 79:61–78. https://doi.org/10.1175/1520-0477(1998)079%3c0061:APGTWA%3e2.0.CO;2
Vargas VP, Cantarella H, Martins AA, Soares JR, Do Carmo JB, De Andrade CA (2014) Sugarcane crop residue increases N2O and CO2 emissions under high soil moisture conditions. Sugar Tech 16:174–179
Vargas VP, Soares JR, Oliveira BG et al (2019) Sugarcane straw, soil temperature, and nitrification inhibitor impact N2O emissions from N fertilizer. Bioenergy Res 12:801–812. https://doi.org/10.1007/s12155-019-10015-8
Vargas VP (2013) Greenhouse gases in soils cropped with sugarcane: fertilization, inoculation, and straw (PhD Thesis). Agronomic Institute (In Portuguese)
Vargha A, Delaney HD (1998) The Kruskal–Wallis test and stochastic homogeneity. J Educ Behav Stat 23:170–192. https://doi.org/10.3102/10769986023002170
Vasconcelos ALS, Cherubin MR, Cerri CEP et al (2022) Sugarcane residue and N-fertilization effects on soil GHG emissions in south-central. Brazil Biomass Bioenergy 158:106342. https://doi.org/10.1016/j.biombioe.2022.106342
Wang WJ, Reeves SH, Salter B et al (2016) Effects of urea formulations, application rates and crop residue retention on N2O emissions from sugarcane fields in Australia. Agric Ecosyst Environ 216:137–146. https://doi.org/10.1016/j.agee.2015.09.035
Yang L, Deng Y, Wang X et al (2021) Global direct nitrous oxide emissions from the bioenergy crop sugarcane (Saccharum spp. inter-specific hybrids). Sci Total Environ 752:141795. https://doi.org/10.1016/j.scitotenv.2020.141795
Yates TT, Si BC, Farrell RE, Pennock DJ (2007) Time, location, and scale dependence of soil nitrous oxide emissions, soil water, and temperature using wavelets, cross-wavelets, and wavelet coherency analysis. J Geophys Res Atmos. https://doi.org/10.1029/2006JD007662
Zeri M, Yang WH, Cunha-Zeri G et al (2020) Nitrous oxide fluxes over establishing biofuel crops: characterization of temporal variability using the cross-wavelet analysis. GCB Bioenergy 12:756–770. https://doi.org/10.1111/gcbb.12728
Acknowledgements
The authors wish to acknowledge funding from São Paulo Research Foundation (FAPESP) grants 2014/26767-9, 2018/20698-6, 2018/20793-9, and 2019/15819-1. For MG and PH gratefully acknowledge the funding provided by the Biotechnology and Biological Sciences Research Council (BBSRC) (BBS/E/C/000J0100, BBS/E/C/000I0320 and BBS/E/C/000I0330), and support from the Growing Health Institute Strategic Programme (BB/X010953/1). GCZ is grateful to the Coordination for the Improvement of Higher Education Personnel (CAPES), grant 88887.308408/2018-00.
Author information
Authors and Affiliations
Contributions
Marcelo V. Galdos: Conceptualization, Formal Analysis, Methodology, Writing- Original draft preparation/Reviewing and Editing. Johnny Soares: Formal Analysis, Visualization, Writing- Original draft preparation/Reviewing and Editing. Kesia Lourenço: Data curation, Formal Analysis. Paul Harris: Methodology, Writing- Reviewing and Editing. Marcelo Zeri: Formal Analysis, Visualization. Gislene Cunha-Zeri: Writing- Original draft preparation/Reviewing and Editing. Victor Vargas: Data curation. Iracema Degaspari: Data curation. Heitor Cantarella: Conceptualization, Funding acquisition, Writing- Reviewing and Editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Galdos, M.V., Soares, J.R., Lourenço, K.S. et al. Multi-experiment assessment of soil nitrous oxide emissions in sugarcane. Nutr Cycl Agroecosyst 127, 375–392 (2023). https://doi.org/10.1007/s10705-023-10321-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10705-023-10321-w