Abstract
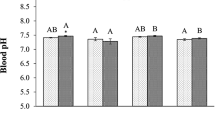
The Indian major carp, mrigal (Cirrhinus mrigala), is a bottom-dwelling fish that can survive hypoxic episodes in its natural environment. We hypothesise that it can better survive hypoxic conditions by altering metabolic responses through GABA (Gamma-aminobutyric acid) supplementation. In the first experiment, the hypoxia tolerance time of the fishes was evaluated under extreme anoxic conditions after feeding with GABA, which showed that GABA had improved survival time under hypoxia. To study the response of dietary GABA in hypoxia-exposed fish, the branchial HIF-1α expression levels, serum thyroid hormone levels and hepatic metabolic responses were assessed in the subsequent experiment. The treatment groups were fed for 60 days with experimental diets containing 4 levels of GABA (0.00% G, 0.50% G, 0.75% G and 1.0%G) and were subjected to 72-h hypoxia exposure (0.5 ± 0.02 mg L−1 dissolved oxygen (DO)) whereas a control group was maintained under normoxic conditions (6.0 ± 0.21 mg L−1 DO). The five treatment groups with three replicates were C0 (0% G + normoxia), H0 (0% G + hypoxia), H0.5 (0.50% G + hypoxia), H0.75 (0.75% G + hypoxia) and H1.0 (1.00% G + hypoxia). The results indicated that GABA supplementation triggered downregulation of HIF 1 alpha expression. When compared with the control group, decreased thyroxine (T4) and triiodothyronine (T3) levels were observed in the GABA-fed hypoxic groups. However, TSH (thyroid stimulating hormone) level remained unchanged in all the treatments. The LDH (lactate dehydrogenase) level in hypoxia-exposed groups was decreased by GABA supplementation. Our study demonstrated that GABA supplementation restores acute hypoxia-induced HIF-1α expression, thyroid hormone levels and LDH activities. On the other hand, it enhanced the citrate synthase (CS) activities at 0.5–1.00%, which showed a sharp decline in hypoxia. Hypoxia caused increase in the serum metabolites such as glucose, lactate, cholesterol and triglycerides. However, GABA supplementation was partially effective in reducing glucose and lactate level while triglycerides and cholesterol values remained unchanged. Overall, our results suggested a potential role of GABA in suppressing metabolism during hypoxia exposure, which can increase the chances of survival of the species Cirrhinus mrigala during hypoxia.



Similar content being viewed by others
References
Abdel-Tawwab M, Monier MN, Hoseinifar SH, Faggio C (2019) Fish response to hypoxia stress: growth, physiological, and immunological biomarkers. Fish Physiol Biochem 1–17
Al Rasheed NM, Fadda L, Mohamed AM, Attia HA, Al Rasheed NM (2016) Regulating effect of carnosine and L-arginine on the expression of inflammatory molecules induced nephropathy in the hypoxic rat model. Braz Arch Biol Technol 59:e16150622
Almeida Val VMF, Farias IP, Paula Silva MN, Duncan WP (1995) Biochemical adjustments to hypoxia by (cf) Amazon Cichlids. Braz J Med Biol Res 28:1257–1263
AOAC (1995) Official methods of analysis of AOAC International, 16 edn. (Eds. Cunniff, P. A.). AOAC International, Arlington, pp 1–201
Barton BA, Schreck CB (1987) Metabolic cost of acute physical stress in juvenile steelhead. Trans Am Fish Soc 116(2):257–263
Brahimi Horn MC, Chiche J, Pouyssegur J (2007) Hypoxia and cancer. J Mol Med 85(12):1301–1307
Brinson BE (2011) Lactate dehydrogenase and Na+/K+ ATPase activity in Leiostomus xanthurus (spot) in response to hypoxia. Explorations 4:22–29
Buentello JA, Gatlin DM, Neill WH (2000) Effects of water temperature and dissolved oxygen on daily feed consumption, feed utilization and growth of channel catfish (Ictalurus punctatus). Aquaculture 182(3):339-352
Burgner JW, Ray WJ Jr (1984) On the origin of the lactate dehydrogenase induced rate effect. Biochemistry 23(16):3636–3648
Carey JB, McCormick SD (1998) Atlantic salmon smolts are more responsive to an acute handling and confinement stress than parr. Aquaculture 168(1):237–253
Chen Z, Tang J, Sun YQ, Xie J (2013) Protective effect of γ-aminobutyric acid on antioxidation function in intestinal mucosa of Wenchang chicken induced by heat stress. J Anim Plant Sci 23(23):1634–1641
Congleton JL, Wagner T (2006) Blood-chemistry indicators of nutritional status in juvenile salmonids. J Fish Biol 69(2):473–490
Connor EAO, Pottinger TG, Sneddon LU (2011) The effects of acute and chronic hypoxia on cortisol, glucose and lactate concentrations in different populations of three-spined stickleback. Fish Physiol Biochem 96:221–237
Cooper RU, Clough LM, Farwell MA, West TL (2002) Hypoxia-induced metabolic and antioxidant enzymatic activities in the estuarine fish Leiostomus xanthurus. J Exp Mar Biol Ecol 279(1):1–20
Craig I (1973) A procedure for the analysis of citrate synthase (EC 4.1. 3.7) in somatic cell hybrids. Biochem Genet 9(4):351–358
Crane RK, Sols A (1955) Animal tissue hexokinases (soluble and particulate forms): Hexose+ ATP → Hexose-6-P+ ADP. Methods Enzymol 1:277–286
Das T, Pal AK, Chakraborty SK, Manush SM, Chatterjee N, Apte SK (2006) Metabolic elasticity and induction of heat shock protein 70 in Labeo rohita acclimated to three temperatures. Asian Australas J Anim Sci 19(7):1033–1039
FAO (Food and Agricultural Organization of the United Nations) (2014) The state of world fisheries and aquaculture. FAO Fisheries and Aquaculture Department, Rome
Ficke AD, Myrick CA, Hansen LJ (2007) Potential impacts of global climate change on freshwater fisheries. Rev Fish Biol Fish 17(4):581–613
Gawehn K, Bergmeyer HU (1974) D-(-)-Lactate. In: Bergmeyer HU (ed) Methods of enzymatic analysis. Academic Press, New York, pp 1492–1495
Getting SJ, Segieth J, Ahmad S, Biggs CS, Whitton PS (1996) Biphasic modulation of GABA release by nitric oxide in the hippocampus of freely moving rats in vivo. Brain Res 717(1):196–199
Gillanders BM, Elsdon TS, Halliday IA, Jenkins GP, Robins JB, Valesini FJ (2011) Potential effects of climate change on Australian estuaries and fish utilising estuaries: a review. Mar Freshw Res 62(9):1115–1131
Gomez R, Asnis N, Tannhauser SL, Barros HM (2001) GABA agonists differentially modify blood glucose levels of diabetic rats. Jpn J Pharmacol 80(4):327–331
Hedrington MS, Tate DB, Younk LM, Davis SN (2015) Effects of antecedent GABA-A receptor activation on counterregulatory responses to exercise in healthy man. Diabetes 64(9):3253–3261
Hochachka PW (1980) Living without oxygen: closed and open systems in hypoxia tolerance. Harvard University Press, Cambridge, p 457
Huang CY, Lin HC, Lin CH (2015) Effects of hypoxia on ionic regulation, glycogen utilization and antioxidative ability in the gills and liver of the aquatic air-breathing fish Trichogaster microlepis. Comp Biochem Physiol A Mol Integr Physiol 179:25–34
Hylland P, Nilsson GE (1999) Extracellular levels of amino acid neurotransmitters during anoxia and forced energy deficiency in crucian carp brain. Brain Res. 823(1-2):49-58
KIM SK, Takeuchi T, Yokoyama M, Murata Y (2003) Effect of dietary supplementation with taurine, β-alanine and GABA on the growth of juvenile and fingerling Japanese flounder Paralichthys olivaceus. Fish Res 69(2):242–248
La VT, Cooke SJ (2011) Advancing the science and practice of fish kill investigations. Rev Fish Sci 19:21–33
Li Z, Yu J, Peng Y, Huang B (2016) Metabolic pathways regulated by γ-aminobutyric acid (GABA) contributing to heat tolerance in creeping bent grass (Agrostiss tolonifera). Sci Rep 6:30338
Lowry OH, Roserbrough NJ, Farr AL, Randall NJ (1951) Estimation of total protein. J Biol Chem 193:265
Lushchak VI (1993) Free and bound pyruvate kinase from fish brain: properties and redistribution after hypoxia. Biochem Mol Biol 29:1103–1109
Manalo DJ, Rowan A, Lavoie T, Natarajan L, Kelly BD, Shui QY, Garcia JG, Semenza GL (2005) Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF-1. Blood 105(2):659–669
Manush SM, Pal AK, Das T, Mukherjee SC (2005) Dietary high protein and vitamin C mitigate stress due to chelate claw ablation in Macrobrachium rosenbergii males. Comp Biochem Physiol A Mol Integr Physiol 142:10–18
Marjorie AS (1964) Estimation of glucose-6-phosphatase. In: Colowick SP, Kaplan NO (eds) Methods in enzymology. Academic Press, New York, p 54
Martinez ML, Landry C, Boehm R, Manning S, Cheek AO, Rees BB (2006) Effects of long-term hypoxia on enzymes of carbohydrate metabolism in the Gulf killifish, Fundulus grandis. Eur J Exp Biol 209(19):3851–3861
Mock A, Peters G (1990) Lysozyme activity in rainbow trout, Oncorhynchus mykiss (Walbaum), stressed by handling, transport and water pollution. J Fish Biol 37(6):873–885
Moore MT, Kroger R, Locke MA, Cullum RF, Steinriede RW, Testa S, Lizotte RE, Bryant CT, Cooper CM (2010) Nutrient mitigation capacity in Mississippi Delta, USA drainage ditches. Environ Pollut 158(1):175–184
Murray AJ (2009) Metabolic adaptation of skeletal muscle to high altitude hypoxia: how new technologies could resolve the controversies. Genome Med 1(12):117
Nilsson GE, Lutz PL (1993) Role of GABA in hypoxia tolerance, metabolic depression and hibernation—possible links to neurotransmitter evolution. Comp Biochem Physiol C Toxicol Pharmacol 105(3):329–336
Nilsson GE, Renshaw GM (2004) Hypoxic survival strategies in two fishes: extreme anoxia tolerance in the North European crucian carp and natural hypoxic preconditioning in a coral-reef shark. J Exp Biol 207(18):3131–3139
O’Connor CM, Gilmour KM, Arlinghaus R, Van Der Kraak G, Cooke SJ (2009) Stress and parental care in a wild teleost fish: insights from exogenous supraphysiological cortisol implants. Physiol Biochem Zool 82(6):709–719
Peeri M, Kohanpour MA, Sanavi S, Pazukian M, Jafarabadi MA, Mirsepasi M (2012) Effects of submaximal aerobic exercise on thyroid hormones in hypoxic conditions in trained young men. Thyroid Res Pract 9(3):88
Perry JC, D’Almeida V, Souza FG, Schoorlemmer GH, Colombari E, Tufik S (2007) Consequences of subchronic and chronic exposure to intermittent hypoxia and sleep deprivation on cardiovascular risk factors in rats. Respir Physiol Neurobiol 156(3):250–258
Peter MS (2011) The role of thyroid hormones in stress response of fish. Gen Comp Endocrinol 172(2):198–210
Pichavant K, Person-Le-Ruyet J, Le Bayon N, Severe A, Le Roux A, Quemener L, Maxime V, Nonnotte G, Boeuf G (2001) Effects of hypoxia on growth and metabolism of juvenile turbot. Aquaculture 188(1):103–114
Pickering AD (1981) Stress and fish. Academic Press, New York, p 361
Rahman MS, Thomas P (2007) Molecular cloning, characterization and expression of two hypoxia-inducible factor-alpha subunits, HIF-1α and HIF-2α, in a hypoxia-tolerant marine teleost, Atlantic croaker (Micropogonias undulatus). Gene 396(2):273–282
Rice JA, Thompson JS, Sykes JA, Waters CT (2013) The role of metalimnetic hypoxia in striped bass summer kills consequences and management implications. In: Bulak JS, Coutant CC, Rice JA (eds) Biology and management of inland striped bass and hybrid striped bass. American Fisheries Society Symposium, U. S. A, pp 1–24
Semenza GL (2000) HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88:1474–1480
Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, Da-Silva WS, Harney J, Engel FB (2008) Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. J Clin Invest 118(3):975–983
Small K, Kopf RK, Watts RJ, Howitt J (2014) Hypoxia, blackwater and fish kills experimental lethal oxygen thresholds in juvenile predatory lowland river fishes. PLoS One 9(4):e94524
Sollid J, De Angelis P, Gundersen K, Nilsson GE (2003) Hypoxia induces adaptive and reversible gross morphological changes in crucian carp gills. J Exp Biol 206(20):3667–3673
Terova G, Rimoldi S, Cora S, Bernardini G, Rosalba G, Saroglia M (2008) Acute and chronic hypoxia affects HIF-1α mRNA levels in sea bass (Dicentrarchus labrax). Aquaculture 279:150–159
Treiman DM (2001) GABAergic mechanisms in epilepsy. Epilepsia 42:8–12
Tripathi RK, Mohindra V, Singh A, Kumar R, Mishra RM, Jena JK (2013) Physiological responses to acute experimental hypoxia in the air-breathing Indian catfish, Clarias batrachus (Linnaeus, 1758). J Biol Sci 38(2):373–383
Varghese S, Shameena B, Oommen OV (2001) Thyroid hormones regulate lipid peroxidation and antioxidant enzyme activities in Anabas testudineus (Bloch). Comp Biochem Physiol B Biochem Mol Biol 128(1):165–171
Varghese T, Pal AK, Sahu NP, Mishal P, Dasgupta S (2017) Effects of hypoxia and dietary vitamin E on growth performance and oxidative status of Cirrhinus mrigala (Ham. 1822). Anim Biol 67(2):133–148
Wedemeyer G (1972) Some physiological consequences of handling stress in the juvenile coho salmon (Oncorhynchus kisutch) and steelhead trout (Salmo gairdneri). J Fish Res Board Can 29(12):1780–1783
Wedemeyer GA, McLeay DJ (1981) Methods for determining the tolerance of fishes to environmental stressors. In: Pickering AD (ed) Stress and fish. Academic Press, New York, pp 247–275
Wedemeyer GA, Barton BA, McLeay DJ (1990) Stress and acclimation. In: Schreck CB, Moyle PB (eds) Methods for fish biology. American Fisheries Society, Bethesda, pp 451–489
Wroblewski L (1955) LDH activity in blood. Proc Soc Exp Biol Med 90:210–213
Yang TH, Somero GN (1993) Effects of feeding and food deprivation on oxygen consumption, muscle protein concentration and activities of energy metabolism enzymes in muscle and brain of shallow-living (Scorpaena guttata) and deep-living (Sebastolobus alascanus) scorpaenid fishes. J. Exp. Biol. 181(1):213-232
Yang H, Tian L, Huang J, Liang G, Liu Y (2013) Dietary taurine can improve the hypoxia-tolerance but not the growth performance in juvenile grass carp Ctenopharyngodon idellus. Fish Physiol Biochem 39(5):1071–1078
Zhang Z, Ju Z, Wells MC, Walter RB (2009) Genomic approaches in the identification of hypoxia biomarkers in model fish species. J Exp Mar Biol Ecol 381:S180–S187
Zhou BS, Wu RSS, Randall DJ, Lam PKS, Chew SF (2000) Metabolic adjustments in the common carp during prolonged hypoxia. J Fish Biol 57:1160–1171
Acknowledgements
The first author is grateful to the Indian Council of Agriculture Research (ICAR)-Central Institute of Fisheries Education (CIFE), for providing necessary facilities and fund for the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research undertaken complies with the current animal welfare laws in India, and the use of animals in this study was in accordance with the guidelines of the CPCSEA ((Committee for the Purpose of Control and Supervision of Experiments on Animals), Ministry of Environment & Forests (Animal Welfare Division), Govt. of India) on care and use of animals in scientific research. The study was undertaken with the approval of statutory authorities of the Central Institute of Fisheries Education, Mumbai, India (University under Sec. 3 of University Grants Commission Act and ISO 9001:2008 certified).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Varghese, T., Rejish Kumar, V.J., Anand, G. et al. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, Cirrhinus mrigala by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol Biochem 46, 199–212 (2020). https://doi.org/10.1007/s10695-019-00708-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-019-00708-4