Abstract
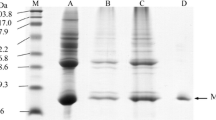
Myoglobin from Asian swamp eel Monopterus albus was purified from fish muscle using salt fractionation followed by column chromatography and molecular filtration. The purified Mb of 0.68 mg/g wet weight of muscle was determined for its molecular mass by MALDI-TOF–MS to be 15,525.18 Da. Using isoelectric focusing technique, the purified Mb showed two derivatives with pI of 6.40 and 7.12. Six peptide fragments of this protein identified by LC–MS/MS were homologous to Mbs of sea raven Hemitripterus americanus, yellowfin tuna Thunnus albacores, blue marlin Makaira nigicans, common carp Cyprinus carpio, and goldfish Carassius auratus. According to the Mb denaturation, the swamp eel Mb had thermal stability higher than walking catfish Clarias batrachus Mb and striped catfish Pangasius hypophthalmus Mb, between 30 and 60 °C. For the thermal stability of Mb, the swamp eel Mb showed a biphasic behavior due to the O2 dissociation and the heme orientation disorder, with the lowest increase in both Kdf and Kds. The thermal sensitivity of swamp eel Mb was lower than those of the other Mbs for both of fast and slow reaction stages. These results suggest that the swamp eel Mb globin structure is thermally stable, which is consistent with heat-tolerant behavior of the swamp eel particularly in drought habitat.










Similar content being viewed by others
References
Awad ES, Deranleau DA (1968) Thermal denaturation of myoglobin. I. Kinetic resolution of reaction mechanism. Biochem 7:1791–1795
Beavis RC, Chait BT (1990) High accuracy molecular mass determination of protein using matrix assisted laser desorption mass spectrometry. Anal Chem 62:1836–1840
Birnbaum GI, Evans SV, Przybylska M, Rose DR (1994) 1.70 Å resolution structure of myoglobin from yellowfin tuna. An example of a myoglobin lacking the D helix. Acta Cryst D50:283–289
Chanthai S, Ogawa M, Tamiya T, Tsuchiya T (1996) Studies on thermal denaturation of fish myoglobins using differential scanning calorimetry, circular dichroism, and tryptophan fluorescence. Fish Sci 62:927–932
Chanthai S, Nieda H, Ogawa M, Tamiya T, Tsuchiya T (1998) Effect of heating on autoxidation rate of fish holo- and reconstituted myoglobins. Fish Sci 64:574–577
Chen HH (2003) Effect of cold storage on the stability of chub and horse mackerel myoglobins. J Food Sci 68:1416–1419
Chen JR, Chang HM (1992) Studies on some shark actomyosins denature factors—I. Food Sci 19:324–335
Chen LC, Lin SB, Chen HH (2004) Thermal stability and denaturation rate of myoglobin from various species of fish. Fish Sci 70:293–298
Chow CJ (1991) Relationship between the stability and autoxidation of myoglobin. J Agric Food Chem 39:22–26
Chow CJ, Wu JC, Lee PF, Ochiai Y (2009) Characterization and kinetics studies of water buffalo (Bubalus bubalis) myoglobin. Comp Biochem Physio Part B 154:274–281
Cossins AR, Williams DR, Foulkes NS, Berenbrink M, Kipar A (2009) Diverse cell-specific expression of myoglobin isoforms in brain, kidney, gill and liver of the hypoxia-tolerant carp and zebrafish. J Exp Biol 212:627–638
Cutruzzolaa F, Travagliniallocatelli C, Brancaccio A, Brunori M (1996) Aplysia limacina myoglobin cDNA cloning: an alternative mechanism of oxygen stabilization as studied by active-site mutagenesis. Biochem J 314:83–90
Dosi R, Maro DA, Chambery A, Colonna G, Costantini S, Geraci G, Parente A (2006) Characterization and kinetics studies of water buffalo (Bubalus bubalus) myoglobin. Comp Biochem Physiol Part B 145:230–238
Fosmire GJ, Brown WD (1976) Yellowfin tuna (Thunnus albacares) myoglobin: characterization and comparative stability. Comp Biochem Physiol 55B:293–299
Fraser J, Mello LV, Ward D, Rees HH, Williams DR, Fang Y, Brass A, Gracey AY, Cossins AR (2006) Hypoxic-inducible myoglobin expression in nonmuscle tissues. Proc Natl Acad Sci USA 103:2977–2981
Hamoir G, Konosu S (1965) Carp Myogens of white and red muscles: general composition and isolation of low-molecular-weight components of abnormal amino acid composition. Biochem J 96:85–96
Helbo S, Dewilde S, Williams DR, Berghmans H, Berenbrink M, Cossins AR, Fago A (2011). Functional differentiation of myoglobin isoforms in the hypoxia-tolerant carp indicates tissue-specific protective roles. Am J Physiol Regul Integr Comp Physiol 302(6):R693–701
Jones NB, Wang CC, Dwulet EF, Lehman DL, Meuth LJ, Bogardt AR, Gurd RNF (1979) Complete amino acid sequence of the myoglobin from the pacific spotted dolphin, Stenella attenuata graffmani. Biochi Biophys Acta Protein Struct 577:454–463
Kendrew JC, Dickerson RE, Strandberg BE, Hart RG, Davies DR, Phillips DC, Shore VC (1960) Structure of myoglobin. Nature 185:422–427
Kooyman GL, Ponganis PJ (1998) The physiological basis of diving to depth: birds and mammals. Annu Rev Physiol 60:19–32
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Light WR, Rohlfs RJ, Palmer G, Olson JS (1987) Functional effects of heme orientational disorder in sperm whale myoglobin. J Biol Chem 262:46–52
Liong EC, Dou Y, Scott EE, Olson JS, Phillips GN Jr (2001) Waterproofing the heme pocket. Role of proximal amino acid side chains in preventing hemin loss from myoglobin. J Biol Chem 276:9093–9100
Marcinek DJ, Bonaventura J, Wittenberg JB, Block BA (2001) Oxygen affinity and amino acid sequence of myoglobins from endothermic and ectothermic fish. Am J Physiol Regul Integr Comp Physiol 280:1123–1133
Marleen BE, Groot L, Tombe LA, Van der Laarse JW (1998) Calibrated histochemistry of myoglobin concentration in cardiomyocytes. J Histochem Cytochem 46:1077–1084
McLellan T (1984) Molecular charge and electrophoretic mobility in cetacean myoglobins of known sequence. Biochem Genet 22:181
Nichols JW, Weber LJ (1989) Comparative oxygen affinity of fish and mammalian myoglobins. J Comp Physiol B 159:205–209
Ochiai Y (2011) Temperature dependent structural perturbation of tuna myoglobin. WASET 74:731–735
Ochiai Y, Watanabe Y, Ozawa H, Ikegami S, Uchida N, Watanabe S (2010) Thermal denaturation profile of tuna. Biosci Biotechnol Biochem 74:1673–1679
Perutz MF, Kendrew JC, Watson HC (1965) Structure and function of haemoglobin II. Some relations between polypeptide chain configuration and amino acid sequence. J Expt Bio J Mol Biol 13:669–678
Polasek LK, Davis RW (2001) Heterogeneity of myoglobin distribution in the locomotory muscles of five cetacean species. J Exp Biol 204:209–215
Satterlee LD, Snyder HE (1964) Isoelectric fractionation of bovine metmyoglobin. J Chromatogr 41:417–422
Schofield JP, Nico GL (2009) Salinity tolerance of non-native Asian swamp eels (Telostei: Synbranchidae) in Florida, USA: comparison of three populations and implication for dispersal. Environ Biol Fish 85:51–59
Seidel ME, Adkins MD (1989) Variation in turtle myoglobins (subfamily emydinae: testudines) examined by isoelectric focusing. Comp Biochem Physiol B 94:569–573
Shareghi H, Nazem Z, Dehkordi KZ, Dehkordi JA (2009) Study on the stability of myoglobin in the presences of sodium dodecyl sulfate (SDS) and temperature. Comp Clin Pathol 19:141–145
Shiraki K, Kudou M, Fujiwara S, Imanakab T, Takagi M (2002) Biophysical effect of amino acids on the prevention of protein aggregation. J Biochem 132:591–595
Siang YH, Yee ML, Seng TC (2007) Acute toxicity of organochlorine insecticide endosulfan and its effect on behaviour and some hematological parameters of Asian swamp eel (Monopterus albus, Zuiew). Pestic Biochem Phys 89:46–53
Stewart JM, Blakely JA, Karpowicz PA, Kalanxhi E, Thatcher BJ, Martin BM (2004) Unusually weak oxygen binding, physical properties, partial sequence, autooxidation rate and a potential phosphorylation site of beluga whale (Delphinapteris leucas) myoglobin. Comp Biochem Physiol B 137:401–412
Sudmoon L, Sattayasai N, Bunyatratchata W, Chaveerach A, Nuchadomrong S (2008) Thermostable mannose-binding lectin from Dendrobium findleyanum with activities dependent on sulfhydryl content. Acta Biochim Biophys Sin 40:811–818
Tay SLA, Chew ST, Ip KY (2003) The swamp eel Monopterus albus reduces endogenous ammonia production and detoxifies ammonia to glutamine during 144 h of aerial exposure. J Exp Biol 206:2473–2486
Ueki N, Ochiai Y (2004) Primary structure and thermostability of bigeye tuna myoglobin in relation to those of other scombridae fish. Fish Sci 70:875–884
Ueki N, Chaujen C, Ochiai Y (2005) Characterization of bullet tuna myoglobin with reference to the thermostability-structure relationship. J Agric Food Chem 53:4968–4975
Watts DA, Rice RH, Brown WB (1980) The primary structure of myoglobin from yellowfin tuna (Thunnus albacares). J Biol Chem 255:10916–10924
Wen LC, Chau JC (2001) Studies on the physicochemical properties of milkfish myoglobin. J Food Biochem 25:157–174
Wittenberg BA, Wittenberg JB, Caldwell PB (1975) Role of myoglobin in the oxygen supply to red skeletal muscle. J Biol Chem 250:9038–9043
Acknowledgments
The research funding supported by Center of Excellence for Innovation in Chemistry (PERCH-CIC) and Rajamangala University of Technology Isan, Thailand, was gratefully acknowledged. Thanks were also extended to the Hitachi Scholarship Foundation, Tokyo, Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chotichayapong, C., Wiengsamut, K., Chanthai, S. et al. Isolation of heat-tolerant myoglobin from Asian swamp eel Monopterus albus . Fish Physiol Biochem 38, 1533–1543 (2012). https://doi.org/10.1007/s10695-012-9644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-012-9644-y