Abstract
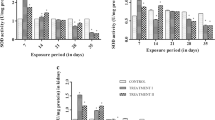
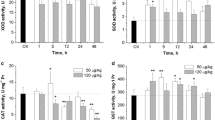
Fish suffer from anemia and hypovolemic hypotensive shock after in vivo exposure with microcystins. However, except for in vivo causes for anemia and hypotension, an in vitro study of fish erythrocytes exposed to MC is necessary. For a better understanding of hematology toxicity of MC, the main aim of the present study was to investigate the toxic effects of microcystin on fish erythrocytes in vitro. Crucian carp erythrocytes were incubated in vitro with microcystin-LR (MC-LR) at doses of 0, 1, 10, 100 and 1,000 nM. The level of lipid peroxidate significantly increased in MC-LR treatment groups. Glutathione decreased after exposure to MC-LR. The activities of antioxidative enzymes, including superoxide dismutase, catalase, glutathione peroxidase and glutathione-S-transferase, were significantly increased after exposure with MC-LR. The hemolysis was significantly increased, while the activities of acetylcholinesterase, Na+–K+-ATPase and Ca2+–Mg2+-ATPase were significantly decreased. In addition, pathological alterations in agglomerated and jagged erythrocytes were observed in blood smears. The findings indicate that damages to erythrocytes should also be responsible for anemia and hypotensive shock or even death.




Similar content being viewed by others
References
Adhikari S, Sarkar B, Chatterjee A, Mahapatra CT, Ayyappan S (2004) Effects of cypermethrin and carbofuran on certain haematological parameters and prediction of their recovery in a freshwater teleost Labeo rohita (Hamilton). Ecotoxicol Environ Saf 58:220–226
Aebi H (1984) Catalase in vitro. In: Packer L (ed) Methods in enzymology, vol 105. Academic Press, Orlando, pp 121–126
Anna A, Zofia J, Teresa G, Roman G (1999) Effect of zinc on carp (Cyprinus carpio L.) erythrocytes. Comp Biochem Physiol C 123:209–215
Bartosz G (1995) The other face of oxygen: in Polish Copyright by PWN Sp. Z o.o, Warszawa, pp 200–204
Beattie KA, Ressler J, Wiegand C, Krause E, Codd GA, Pflug-macher S (2003) Comparative effects and metabolism of two microcystins and nodularin in the brine shrimp Artemia salina. Aquat Toxicol 62:219–226
Beutler E (1975) Red cell metabolism. A manual of biochemical methods, 2nd edn. Grune & Stratton, New York, pp 71–73
Bukowska B, Kowalska S (2004) Phenol and catechol induce prehemolytic and hemolytic changes in human erythrocytes. Toxicol Lett 152:73–84
Bukowska B, Zatorska A (2003) Prehemolytical changes in erythrocytes incubated with 2, 4-d. Curr Topics Biophys 27:11–15
Cazenave J, Bistoni MA, Pesce SF, Wunderlinm DA (2006) Differential detoxification and antioxidant response in diver se organs of Corydoras paleatus experimentally exposed to microcystin-RR. Aquat Toxicol 76:1–12
Chauhan VPS, Tsiouris JA, Chauhan A, Sheikh AM, Brown WT, Vaughan M (2002) Increased oxidative stress and decreased activities of Ca2+/Mg2+-ATPase and Na+/K+-ATPase in the red blood cells of the hibernating black bear. Life Sci 71:153–161
Chiu D, Lubin B (1989) Oxidative hemoglobin denaturation and RBC destruction: the effect of heme on red cell membranes. Semin Hematol 26:128–135
Clemens MR, Remmer H, Waller HD (1984) Phenylhydrazine-induced lipid peroxidation of red blood cells in vitro and in vivo: monitoring by the production of volatile hydrocarbons. Biochem Pharmacol 333:1715–1718
Cohen P (1989) The structure and regulation of protein phosphatases. Annu Rev Biochem 58:435–508
Dacie JV, Lewis SM (1984) Practical haematology. Churchill Livingstone, New York, p 32
Ellman GL, Courtney KD, Andres VJ, Featherstone RM (1961) A new rapid colorimetric determination of acetylcholine esterase activity. Biochem Pharmacol 7:88–94
Fastner J, Codd GA, Metcalf JS, Woitke P, Wiedner C, Utkilen H (2002) An international intercomparison exercise for the determination of purified microcystin-LR and microcystins in cyanobacterial field material. Anal Biochem Chem 374:437–444
French JK, Winterbourn CC, Carrel RW (1978) Mechanism of oxyhaemoglobin breakdown on reaction with acetylphenylhydrazine. Biochem J 173:19–26
Greenburg AG (1996) Pathophysiology of anemia. Am J Med 101(Suppl. 2A):7S–11S
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferase. J Biol Chem 249:7130–7139
Hebbel RP, Eaton JW, Balasingan M, Steinberg MH (1982) Spontaneous oxygen radical generation by sickle erythrocytes. J Clin Invest 79:1254–1260
Ji LL (1999) Antioxidants and oxidative stress in exercise. Proc Soc Exp Biol Med 222:283–292
Kale M, Rathore N, John S, Bhatnagar D (1999) Lipid peroxidative damage on pyrethroid exposed and alterations in antioxidant status in rat erythrocytes: a possible involvement of reactive oxygen species. Toxicol Lett 105:197–205
Lawrence A, Burk RF (1976) Glutathione peroxidase activity in selenium deficient rat liver. Biochem Biophys Res Commun 71:952–958
Lei H, Xie P, Chen J, Liang G, Dai M, Zhang X (2008) Distribution of toxins in various tissues of crucian carp intraperitoneally injected with hepatotoxic microcystins. Environ Toxicol Chem 27:1167–1174
Li D, Xie P, Zhang X, Zhao Y (2009) Intraperitoneal injection of extracted microcystins results in hypovolemia and hypotension in crucian carp (Carassius auratus). Toxicon 53:638–644
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with Folin phenol reagent. J Biol Chem 193:265–275
Maiti PK, Kar A (1997) Dual dose of testosterone in fenvalerate-treated mice with respect to thyroid function and lipid peroxidation. J Appl Toxicol 17:127–131
Malbrouck C, Kestemont P (2006) Effects of microcystins on fish. Environ Toxicol Chem 25:72–86
Malgorzata W, Barbara J, Jacek W (2005) Respiratory and hematological response of tench, Tinca tinca (L.) to a short-term cadmium exposure. Aquacult Int 14:141–152
Mates JM (2000) Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 153:83–104
McCord JM, Fridovich I (1969) Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem 244:6049–6055
Neefjes VM, Evelo CT, Baars LG, Blanco CE (1999) Erythrocyte glutathione S transferase as a marker of oxidative stress at birth. Arch DisChild-Fetal 81:130–133
Nishiwaki-Matsushima R, Ohta T, Nishiwaki S, Suganuma M, Kohyama K, Ishikawa T, Carmichael WW, Fujiki H (1992) Liver tumor promotion by the cyanobacterial cyclic peptide toxin microcystin-LR. J Cancer Res Clin 118:420–424
Ohta A, Mohri T, Ohyashiki S (1989) Effect of LPO on membrane bound Ca2+ATPase activity of the intestinal brush border membrane. Biochim Biophys Acta 984:151–157
Orhan H, Sahin G (2001) In vitro effects of NSAIDS and paracetamol on oxidative stress-related parameters of human erythrocytes. Exp Toxicol Pathol 53:133–140
Paerl HW, Fulton RS, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World 1:76–113
Paulina S, Bozena B, Jaromir M, Wirgiliusz D (2006) Damage of cell membrane and antioxidative system in human erythrocytes incubated with microcystin-LR in vitro. Toxicon 47:387–397
Pflugmacher S, Wiegand C, Oberemm A, Beattie KA, Krause E, Codd GA, Steingerg CEW (1998) Identification of an enzymatically formed glutathione conjugate of the cyanobacterial hepatotoxin microcystin-LR : the first step of detoxication. Biochim Biophys Acta 1425:527–533
Prieto AI, Jos A, Pichardo S, Moreno IM, Caméan AM (2006) Differential oxidative stress responses to microcystins LR and RR in intraperitoneally exposed tilapia fish (Oreochromis sp.). Aquat Toxicol 77:314–321
Prieto AI, Pichardo S, Jos Á, Moreno IM, Caméan AM (2007) Time-dependent oxidative stress responses after acute exposure to toxic cyanobacterial cells containing microcystins in tilapia fish (Oreochromis niloticus) under laboratory conditions. Aquat Toxicol 84:337–345
Robinson NA, Miura GA, Matson CF, Lawrence WB, Pace JC (1989) Characterization of chemically tritiated microcystin-LR and its distribution in mice. Toxicon 27:1035–1042
Šetlíková I, Wiegand C (2009) Hepatic and branchial glutathione S-transferases of two fish species: Substrate specificity and biotransformation of microcystin-LR. Comp Biochem Physiol C 149:515–523
Stock J, Dormandy TL (1971) The autooxidation of human red cell lipid induced hydrogen peroxide. Br J Haematol 20:95–101
Su MQ, Kinoshita FK, Frawley JP, Dubois KP (1971) Comparative inhibition of aliesterases and cholinesterase in rats fed eighteen organophosphorus insecticides. Toxicol Appl Pharmacol 20:241–249
Üner N, Oruç EO, Sevgiler Y, Sahin N, Durmaz H, Usta D (2006) Effects of diazinon on acetylcholinesterase activity and lipid peroxidation in the brain of Oreochromis niloticus. Environ Toxicol Pharmacol 21:241–245
Vaddi DR, Pannuru P, Maturu P (2009) Modulatory role of Emblica officinalis against alcohol induced biochemical and biophysical changes in rat erythrocyte membrane. Food Chem Toxicol 47:1963–1985
Valenzeno DP (1987) Photomodification of biological membranes with emphasis on singlet oxygen mechanisms. Photochem Photobiol 46:147–160
Vosyliene MZ (1999) The effect of heavy metals on haematological indices of fish (survey). Acta Zool Lituanica 9:76–82
Wang YM, Peng SQ, Zhou Q, Wang MW, Yan CH, Wang GQ, Yang HY (2007) The oxidative damage of butenolide to isolation erythrocytes membranes. Toxicol In Vitro 21:863–869
Yang ZP, Dettbarn WD (1996) Diisopropylphosphorofluoridate induced cholinergic hyperactivity and lipid peroxidation. Toxicol Appl Pharmacol 138:48–53
Zeni C, Bovolenta MR, Stagni A (2002) Occurrence of echinocytosis in circulating RBC of black bullhead, Ictalurus melas (Rafinesque), following exposure to an anionic detergent at sublethal concentrations. Aquat Toxicol 57:217–224
Zhang X, Xie P, Li D, Shi Z (2007) Haematological and plasma biochemical responses of crucian carp (Carassius auratus) to intraperitoneal injection of extracted microcystins with the possible mechanisms of anemia. Toxicon 49:1150–1157
Zhang X, Xie P, Wang W, Li D, Li L, Tang R, Lei H, Shi Z (2008) Dose dependent effects of extracted microcystins on embryonic development, larval growth and histopathological changes of southern catfish Silurus meridionalis. Toxicon 51:449–456
Acknowledgments
We would like to express our sincere thanks to Dr. Patrick Kestemont and anonymous reviewers for their useful comments and suggestions on the manuscript. This study is funded by the National Natural Science Foundation of China (No. 30800160) and the Natural Science Foundation for Distinguished Young Scholars of Hubei Province (No. 2010CDA095).
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenshan Zhou and Hualei Liang have equally contributed to this work.
Rights and permissions
About this article
Cite this article
Zhou, W., Liang, H. & Zhang, X. Erythrocyte damage of crucian carp (Carassius auratus) caused by microcystin-LR: in vitro study. Fish Physiol Biochem 38, 849–858 (2012). https://doi.org/10.1007/s10695-011-9572-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-011-9572-2