Abstract
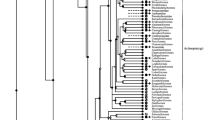
AMP-deaminase (AMPD, EC 3.5.4.6), which catalyzes the irreversible hydrolytic deamination of AMP to IMP and ammonia, is an important energy-related enzyme. The partial genomic sequence of the gene encoding myoadenylate deaminase (AMPD1) from the teleost fish Platichthys flesus was determined. The amino acid sequence of P. flesus AMPD1 shows 82% homology with that of the teleost fish Danio rerio. Comparison of genomic sequences of P. flesus and Rattus norvegicus reveals a high degree of conservation of both sequence and structural organization. A phylogenetic analysis of AMPD sequences shows that bony fish and mammalian AMPD1s arise by duplication of a common primordial gene.


Similar content being viewed by others
References
Bausch-Jurken MT, Sabina RL (1995) Divergent N-terminal regions in AMP deaminase and isoform-specific catalytic properties of the enzyme. Arch Biochem Biophys 321:372–380
Cossins AR, Crawford DL (2005) Fish as models for environmental genomics. Nat Rev Genet 6:324–333
Dayhoff MO (1979) Atlas of protein sequence and structure, vol 5. National Biomedical Research Foundation, Silver Springs, ND
Han BW, Bingman CA, Mahnke DK, Bannen RM, Bednarek SY, Sabina RL, Phillips GN Jr (2006) Membrane association, mechanism of action and structure of Arabidopsis embryonic factor 1 (FAC1). J Biol Chem 281:14939–14947
Hancock CR, Brault JJ, Terjung RL (2006) Protecting the cellular energy state during contractions: role of AMP deaminase. J Physiol Pharmacol 57:17–29
Kaletha K, Thébault MT, Raffin JP (1991) Comparative studies on heart and skeletal muscle AMP-deaminase from rainbow trout (Salmo gairdneri). Comp Biochem Physiol 99B:751–754
Mahnke-Zizelman DK, Sabina R (1992) Cloning of human AMP deaminase isoform E cDNAs. Evidence for a third AMPD gene exhibiting alternatively spliced 5′-exons. J Biol Chem 267:20866–20877
Marchand J, Quiniou L, Riso R, Thébault MT, Laroche J (2004) Physiological cost of tolerance to toxicants in the European flounder Platichthys flesus, along the Atlantic coast of France. Aquat Toxicol 70:327–343
Morisaki T, Sabina RL, Holmes EW (1990) Adenylate deaminase. A multigene family in humans and rats. J Biol Chem 265:11482–11486
Raffin JP, Leray C (1980) Comparative study on AMP deaminase in gill, muscle and blood of fish. Comp Biochem Physiol 67B:533–540
Raffin JP, Thébault MT (1991) A specific AMP deaminase assay and its application to tissue homogenates. Comp Biochem Physiol 99B:125–127
Raffin JP, Thébault MT (1994) Amplification of myoadenylate deaminase during evolution. A comparative study. CR Acad Sci 317:386–391
Sabina RL, Mahnke-Zizelman DK (2000) Towards an understanding of the functional significance of N-terminal domain divergence in human AMP deaminase isoforms. Pharmacol Ther 87:279–283
Sabina RL, Morisaki T, Clarke P, Eddy R, Shows TB, Morton CC, Holmes E (1990) Characterization of the human and rat myoadenylate deaminase genes. J Biol Chem 265:9423–9433
Solliti P, Merkler DJ, Estupinan B, Schramm VL (1993) Yeast AMP deaminase. Catalytic activity in Schizosaccharomyces pombe and chromosomal location in Saccharomyces cerevisiae. J Biol Chem 268:4549–4555
Stankiewicz A (1986) Non specific snail muscle adenylate deaminase: simplified purification, characterization and use for the preparation of deamino derivates of NAD, NADH and AMP-P(NH)P. Enzyme 36:187–196
Takahashi K, Rooney AP, Nei M (2000) Origins and divergence times of mammalian class IIMHC gene clusters. Heredity 91:198–204
Thébault MT, Izem L, Leroy JP, Gobin E, Charrier G, Raffin JP (2005) AMP-deaminase in elasmobranch fish: a comparative histochemical and enzymatic study. Comp Biochem Physiol 141B:472–479
Tscharntke T, Hochberg ME, Rand TA, Resh VH, Krauss J (2007) Author sequence and credit for contributions in multiauthored publications. PloS Biol 5:13–14
Turner DH, Turner JF (1961) Adenylic deaminase of pea seeds. Biochem J 79:143–147
Van den Berghe G, Bontemps F, Vibcebt MF, Van den Berghe F (1992) The purine nucleotide cycle and its molecular defects. Prog Neurobiol 39:547–561
Van Waarde A, Kesbeke F (1981) Regulatory properties of AMP-deaminase from lateral red muscle and dorsal white muscle of goldfish, Carassius auratus (L). Comp Biochem Physiol 69B:413–423
Acknowledgments
We would like to thank M.S. M’Boumba for her excellent technical assistance. We applied the FLAE (first–last-author emphasis) approach combined with EC (equal contribution): + (1st and 2nd authors) for the sequence of authors (Tscharntke et al. 2007).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thébault, M.T., Tanguy, A., Meistertzheim, A.L. et al. Partial characterization of the gene encoding myoadenylate deaminase from the teleost fish Platichthys flesus . Fish Physiol Biochem 36, 819–825 (2010). https://doi.org/10.1007/s10695-009-9358-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-009-9358-y