Abstract
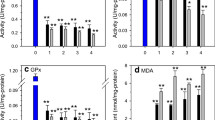
Many ultraviolet-A (UVA)-induced biochemical and physiological changes are valid as biomarkers using aquatic species for detection of the degree of stress. Changes in the concentration and activities of enzymes, such as glucose-6-phosphate dehyderogenase (G6PDH), lactate dehyderogenase (LDH), DNA damage and lipid peroxidation (LPO), can be used as biomarkers to identify possible environmental contamination in fish. This study aimed to investigate the impact of UVA on the activity of the selected enzymes, DNA damage and LPO during early developmental stages of the African catfish Clarias gariepinus. Embryo hemogenates were used for measurements of G6PDH, LDH, DNA damage and LPO concentrations and activities spectrophotometrically at 37°C. The normal ontogenetic variations in enzyme activities, DNA damage and LPO of the early developmental stages (24–168 h-PFS; hours-post fertilization stage) were studied. There was a significant decrease in the activity of G6PDH till 120 h-PFS. Then after 120 h-PFS, the activity of such enzymes insignificantly increased toward higher stages. The LDH activity was recorded with a pattern of decrease till 96 h-PFS, followed by a significant increase toward 168 h-PFS. The polynomial pattern of variations in DNA damage and LPO was also evident. The patterns of the enzyme activities, corresponding DNA damage and LPO of the early ontogenetic stages under the influence of three different UVA doses (15, 30 and 60 min), were recorded. The pattern of variations in G6PDH activity in UVA-induced groups was similar to that of the control group with variation in the magnitude of such activity. In all treated groups, LDH activity decreased till 96 h-PFS, then increased till 168 h-PFS. Within each of the embryonic stages, the increase in UVA led to a significant increase in DNA damage. A significant increase in lipid peroxidation under UVA doses was recorded. The variability in number and molecular weight of proteins under exposure to UVA was evident, reflecting some of the genetic and transcriptional changes during exposure and development.










Similar content being viewed by others
References
Alapetite C, Wachter T, Sage E, Moustacchi E (1996) Use of alkaline comet assay to detect DNA repair deficiencies in human fibroblasts exposed to UVC, UVB, UVA and gamma-rays. Int J Radiat Biol 69:359–369. doi:10.1080/095530096145922
Almeida JA, Novelli ELB, Silva MD, Alves R (2001) Environmental cadmium exposure and metabolic responses of the Nile tilapia, Oreochromis niloticus. Environ Pollut 114:169–175
Andrew TEH, Jacob GS (2003) Epithelial activity of hexokinase and glucose-6-phosphate dehydrogenase in cultured bovine lenses recovering from pharmaceutical-induced optical damage. Mol Vis 9:594–600
Ankley GT, Collyard SA, Monson PD, Kosian PA (1994) Influence of ultraviolet light on the toxicity of sediment contaminated with polycyclic aromatic hydrocarbons. Environ Conta Toxicol 13:1791–1796
Annie M, Marquis I, Gaboriau F, Santus R, Dubertret L, Morlière P (1993) Ultraviolet A-induced lipid peroxidation and antioxidant defense systems in cultured human skin fibroblasts. J Invest Dermatol 100:692–698. doi:10.1111/1523-1747.ep12472352
Armeni T, Damiani E, Battino M, Greci L, Principato G (2004) Lack of in vitro protection by a common sunscreen ingredient on UVA-induced cytotoxicity in keratinocytes. Toxic 203:165–178. doi:10.1016/j.tox.2004.06.008
Armstrong TN, Reimschuessel R, Bradly BP (2002) DNA damage, histological changes and DNA repair in larval Japanese medaka (Oryzias latipes) exposed to ultraviolet-B radiation. Aquat Toxicol 58:1–14
Berges JA, Ballantyne JS (1991) Size scaling of whole body maximal enzyme activities in aquatic crustaceans. Can J Fish Aquac Sci 48:2385–2394
Brian LD (2002) Sources and measurements of ultraviolet radiation. Methods 28(1):4–13. doi:10.1016/S1046-2023(02)00204-9
Browman HI, Vetter RD, Flodriguez CA, Cullen JJ, Davis RF, Lynn E, Pierre J (2003) Ultraviolet (280–400 nm)-induced DNA damage in the eggs and larvae of Calanus finmarchicus G. (Copepoda) and atlantic cod (Gadus morhua). Photochem Photobiol 77(4):397–404
Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Methods Enzymol 52:302–310. doi:10.1016/S0076-6879(78)52032-6
Castro-Pérez CA (2004) Effects of ultraviolet radiation exposure on the swimming performance and hematological parameters of tambaqui, colossoma macropomum. International Congress on the Biology of Fish, Tropical Hotel Resort, Manaus Brazil, August: 1–5
Ciereszko A, Tobie DW, Konrad D (2005) Analysis of DNA damage in sea lamprey (Petromyzon marinus) spermatozoa by UV, hydrogen peroxide, and the toxic antbisazir. Aquat Toxicol 73(2):128–138
Clarke ME, Calvi C, Domeier M, Edmonds M, Walsh PJ (1992) Effects of nutrition and temperature on metabolic enzyme activities in larval and juvenile red drum, Sciaenops ocellatus and lane snapper, Lutjanus synagris. Marine Biol 112:31–36
Clydesdale GJ, Dandie GW, Muller HK (2001) Ultraviolet light induced injury: Immunological and inflammatory effects. Immunol Cell Biol 79(6):547–568. doi:10.1046/j.1440-1711.2001.01047.x
Coquelle N, Fioravanti E, Weik M, Vellieux F, Madern D (2007) Activity, stability and structural studies of lactate dehydrogenases adapted to extreme thermal environments. J Mol Biol 374:547–562
De Graaf GJ, Janssen H (1996) Artificial reproduction and pond rearing of the African catfish Clarias gariepinus in sub-Saharan Africa. FAO, Fish Tech Pap 362:1–73
Degroot SJ (1987) Culture of Clarias species. Aquaculture 63:1–36. doi:10.1016/0044-8486(87)90056-1
Dong Q, Svoboda K, Tiersch TR, Monroe WT (2007) Photobiological effects of UVA and UVB light in zebrafish embryos: evidence for a competent photorepair system. J Photochem Photob B Biol 88:137–146. doi:10.1016/j.jphotobiol.2007.07.002
Douki T, Reynaud-Angelin A, Cadet J, Sage E (2003) Bipyrimidine photoproducts rather than oxidative lesions are the main type of DNA damage involved in the genotoxic effect of solar UVA radiation. Biochem 42:9221–9226. doi:10.1021/bi034593c
Dovrat A, Weinreb O (1995) Recovery of lens optics and epithelial enzymes after ultraviolet A radiation. Invest Ophthalmol Vis Sci 36:12
Elumalai M, Antunes C, Guilhermino L (2002) Effects of single metals and their mixtures on selected enzymes of Carcinus maenas. Water Air Soil Pollut 141:273–280
Estey T, Cantore M, Weston PA, Carpenter JF, Petrash JM, Vasiliou V (2007) Mechanisms involved in the protection of UV-induced protein inactivation by the corneal crystallin ALDH3A1. J Biol Chem 282(7):4382–4392
Fu Y, Jin X, Wei S, Lin H, Kacew S (2000) Ultraviolet radiation and reactive oxygen generation as inducers of keratinocyte apoptosis: protective role of tea polyphenols. J Toxicol Environ Health 61(3):177–188
Gaboriau F, Morliére P, Marquis I, Moysan A, Géze M, Dubertret L (1993) Membrane damage induced in cultured human skin fibroblasts by UVA irradiation. Photochem Photobiol 58:515–520. doi:10.1111/j.1751-1097.1993.tb04924.x
Gallagher RP, Lee TK (2006) Adverse effects of ultraviolet radiation: a brief review. Prog Biophys Mol Biol 92:119–131. doi:10.1016/j.pbiomolbio.2006.02.011
Gantchev TG, van Lier JE (1995) Catalase inactivation following photosensitization with tetrasulfonated metallophtalocyanines. Photochem Photobiol 62:123–134. doi:10.1111/j.1751-1097.1995.tb05248.x
Gornall AC, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the Biuret reaction. J Biol Chem 177:751–766
Häkkinen J, Oikari A (2004) A field methodology to study effects of UV radiation on fish larvae. Water Res 38:2891–2897
Jarvis RB, Knowles JF (2003) DNA damage in zebrafish larvae induced by exposure to low-dose rate gamma-radiation: detection by the alkaline comet assay. Mutat Res Genet Toxicol Environ Mutagen 541:63–69
Jochen G, Lidia L, Hubert T, Ute R, Claus U (1996) Isolation, sequencing and overproduction of the single-stranded DNA binding protein from Pseudomonas aeruginosa PAO. Gene 182:137–143. doi:10.1016/S0378-1119(96)00535-5
Kachmar JF, Moss DW ( 1976) In: Tietz NW (ed) Fundamentals of clinical chemistry, 2nd edn. WB Saunders, Philadelphia, p 652
Kevin RA (1994) Impact of ozone depletion on phytoplankton growth in the Southern Ocean: large-scale spatial and temporal variability. Mar Ecolo Prog 114:1–12. doi:10.3354/meps114001
Kielbassa C, Len R, Bernd E (1997) Wavelength dependence of oxidative DNA damage induced by UV and visible light. Carcinogenesis 18:811–816
Kino K, Sugiyama H (2005) UVR-induced G–C to C–G transversions from oxidative DNA damage. Mutat Res 571:33–42
Kligman LH, Akin FJ, Kligman AM (1983) Sunscreens promote repair of ultraviolet radiation-induced dermal damage. J Investig Dermatol 81:98–102
Kornberg A (1955) Lactic dehydrogenase of muscle. In: Lowestein JM (ed) Methods in enzymology. Academic Press, New York, pp 441–442
Kurita-Ochiai T, Fukushima K, Ochiai K (1999) Lipopolysaccharide stimulates butyric acid-induced apoptosis in human peripheral blood mononuclear cells. Infect Immun 67:22–29
Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227: 680–685
Lee SM, Koh H, Park D, Song BJ, Huh T, Park J (2002) Cytosolic NADP+-dependent isocitrate dehydrogenase status modulates oxidative damage to cells. Free Radic Biol Med 32(11):1185–1196
Legrand C, Bour JM, Jacob C, Capiaumont J, Martial J, Marc A, Wudtke M, Kretzmer G, Demangel C, Duval D, Hache J (1992) Lactate dehydrogenase (LDH) activity of the number of dead cells in the medium of cultured eukaryotic cells as marker. J Biotechnol 25:231–243
Lemos D, Salomon M, Gomes V, Phan V, Buchholz E (2003) Citrate synthase and pyruvate kinase activities during early life stages of the shrimp Farfantepenaeus paulensis (Crustacea, Decapoda, Penaeidae): effects of development and temperature. Comp Biochem Physiol B 135:707–719
Little EE, Cleveland L, Hurtubise R, Barron MG (2000) Assessment of the photoenhanced toxicity of a weathered petroleum to the tidewater silverside. Environ Toxicol Chem 19:926–932. doi:10.1897/1551-5028(2000)019<0926:AOTPTO>2.3.CO;2
Losey GS, Hydes D (1998) The UV visual world of fishes. J Fish Biol 54:921–945. doi:10.1111/j.1095-8649.1999.tb00848.x
Mallakin A, McConkey BJ, Miao G, Mckibben B, Snieckus V, Dixon DG, Greenberg BM (1999) Impacts of structural photomodification on the toxicity of environmental contaminants: anthracene photooxidation products. Ecotoxic Envir saf 43:204–212
McCloskey JT, Oris JT (1993) Effect of anthracene and solar ultraviolet radiation exposure on gill ATPase and selected hematologic measurements in the bluegill sunfish (Lepomis macrochirus). Aquat Toxicol 24:207–218. doi:10.1016/0166-445X(93)90072-9
McFadzen I, Baynes S, Hallam J, Beesley A, Lowe D (2000) Histopathology of the skin of UV-B irradiated sole (Solea solea) and turbot (Scophthalmus maximus) larvae. Marine Environ Res 50:273–277
Mekkawy IAA, Lashein FE (2003) The effect of lead and cadmium on LDH and G-6-PDH isozyme patterns exhibited during the early embryonic development of the teleost fish, Ctenopharyngodon idellus with emphasis on the corresponding morphological variations. The Big Fish Bang, the proceeding of the 26th Annual Larval Fish Conference (LFC2002), 22-26 July, 2002, Bergen, Norway, edited by: Howard I. Browman and Anne Berit Skiftesvik
Merwald H, Klosner G, Kokesch C, Der-Petrossian M, Honigsmann H, Trautinger F (2005) UVA-induced oxidative damage and cytotoxicity depend on the mode of exposure. J Photochem Photob B Biol 79(3):197–207. doi:10.1016/j.jphotobiol.2005.01.002
Morlière P, Annie M, René S, Gabriele H, Jean-Claude M, Louis D (1991) UVA-induced lipid peroxidation in cultured human fibroblasts. BBA Lipids Lipid Metab 1084(3):261–268
Nathanailides C (1996) Metabolic specialization of muscle during development in cold water and warm water fish species exposed to different thermal conditions. Can J Fish Aquat Sci 53:2147–2155
Nguyen LTH, Janssen CR (2002) Embryo-larval toxicity tests with the African catfish (Clarias gariepinus): comparative sensitivity of endpoints. Arch Environ Contam Toxicol 42:256–262. doi:10.1007/s00244-001-0007-4
Obermüller B, Puntarrulo S, Abele D (2007) UV-tolerance and instantaneous physiological stress responses of two Antarctic amphipod species Gondogeneia antarctica and Djerboa furcipes during exposure to UV radiation. Mar Environ Res 64:267–285. doi:10.1016/j.marenvres.2007.02.001
Osman AG, Mekkawy IAA, Verreth J, Kirschbaum F (2007a) Effects of lead nitrate on the activity of metabolic enzymes during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol Biochem 33:1–13. doi:10.1007/s10695-006-9111-8
Osman AG, Wuertz S, Mekkawy IAA, Exner H, Kirschbaum F (2007b) Lead induced malformations in embryos of the African catfish Clarias gariepinus (Burchell, 1822). Environ Toxicol 22(4):375–389. doi:10.1002/tox.20272
Osman AG, Mekkawy IAA, Verreth J, Wuertz S, Kloas W, Kirschbaum F (2008) Monitoring of DNA breakage in embryonic stages of the African catfish Clarias gariepinus (Burchell, 1822) after exposure to lead nitrate using alkaline comet assay. Environ Toxicol 23:679–687. doi:10.1002/tox.20373
Pandey S, Parvez S, Sayeed I, Haque R, Bin-Hafeez B, Raisuddin S (2003) Biomarkers of oxidative stress: a comparative study of river Yamuna fish Wallago attu (Bl. & Schn.). Sci Total Environ 309:105–115
Pelletier D, Blier PU, Dutil JD, Guderley H (1995) How should enzyme activities be used in fish growth studies? J Exp Biol 198:1493–1497
Petersen AB, Gniadecki R, Vicanova J, Thorn T, Wulf HC (2000) Hydrogen peroxide is responsible for UVA-induced DNA damage measured by alkaline comet assay in HaCaT keratinocytes. J photo photob B Biology 59:123–131
Punnonen K, Puntala A, Ansen CT, Ahotupa M (1991) UVB irradiation induces lipid peroxidation and reduced antioxidant enzyme activities in human keratinocytes in vitro. Acta Dermato-venerologica 1:239–273
Rosety-Rodriguez M, Ordonez F, Rosety I, Rosety J, Rosery M (2005) Erythrocyte antioxidant enzymes of gilthead as early-warning bio-indicators of oxidative stress induced by malathion. Haematology 8:237–240
Rozema J, Van Geel B, Bjorn LO, Lean J, Madronich S (2002) Toward solving the UV puzzle. Science 296:1621–1622. doi:10.1126/science.1070024
Salo H, Jokinen E, Markkula S, Aaltonen T, Penttilä H (2000) Comparative effects of UVA and UVB irradiation on the immune system of fish. J photo photob B. Biology 56:154–162
Sastry KV, Gupta PK (1980) Alterations in the activities of a few dehydrogenases in the digestive system of 2 teleost fishes exposed to lead nitrate. Ecotoxicol Environ Saf 4:232–239
Segner H, Verreth J (1995) Metabolic enzyme activities in larvae of the African catfish, Clarias gariepinus—changes in relation to age and nutrition. Fish Physiol Biochem 14:385–398
Setlow RB, Woodhead AD (1994) Temporal changes in the incidence of malignant melanoma: explanation from action spectra. Mutat Res 307:365–374. doi:10.1016/0027-5107(94)90310-7
Setlow RB, Gist E, Thompson K, woodhead Avril D (1993) Wavelengths effective in induction of malignant melanoma. Genetics 90:6666–6670
Singh RK, Sharma B (1998) Carbofuran-induced biochemical changes in Clarias batrachus. Pest Sci 53:285–290
Singh SS, Pankaj k, Ashwani k (2006) Ultraviolet radiation stress: molecular and physiological adaptations in trees in A biotic stress tolerance in plants Springer etherlands editer. doi:10.1007/14020-4389
Sinha RP, Häder D (2002) UV-induced DNA damage and repair: a review. Photochem Photobiol Sci 1:225–236
Societe Francaise de Biologie Clinique (1982) Enzymology commission. Recommendations. Ann Biol Clin (Paris) 40:87–164
Somero S, Childress J (1985) Scaling of oxidative and glycolytic enzyme activities in fish muscle. Springer, Berlin
Stephensen E, Svavarsson J, Sturve J, Ericson G, Adolfsson-Erici M, Förlin L (2000) Biochemical indicators of pollution exposure in shorthorn sculpin (Myoxocephalus scorpius), caught in four harbours on the southwest coast of Iceland. Aquat Toxicol 48:431–442
Thomas E, Ursula F, Rainhardt O (2001) DNA damage and apoptosis in mononuclear cells from glucose-6-phosphate dehydrogenase-deficient patients (G6PD Aachen variant) after UV irradiation. J Leukoc Biol 69:340–342
Tsubai T, Matsuo M (2002) Ultraviolet light-induced changes in the glucose 6-phosphate dehydrogenase activity of porcine corneas. Cornea 21(5):495–500. doi:10.1097/00003226-200207000-00011
Utley HC, Bernheim F, Hachslien P (1967) Effects of sulfhydryl reagent on peroxidation in microsome. Arch Biochem Biophys 260:521–531
Volckaert FAM, Hellemans BA, Galbusera P, Ollevier F, Sekkali B, Belayew A (1994) Replication, expression and fate of foreign DNA during embryonic and larval development of the African catfish Clarias gariepinus. Mol Mar Biol Biotechnol 3:57–69
Weatherhead EC, Stevermer A (2001) Ultraviolet radiation. In: Encyclopedia of global environmental change, vol 1. John Wiley and Sons Inc
Weatherhead EC, George CT, Gregory CR, John EF, John JD, Dongseok C (1997) Analysis of long-term behavior of ultraviolet radiation measured by Robertson-Berger meters at 14 sites in the United States. J Geophys Res 102(d7):8737–8754. doi:10.1029/96JD03590
Weatherhead SC, Haniffa M, Lawrence CM (2007) Melanomas arising from naevi and de novo melanomas does origin matter? Briti J derma 156:72–86
WHO (2003) Climate change and human health—risks and responses—summary. World Health Organization, Geneva
Williamson CE (1995) What role does UV-B radiation play in freshwater ecosystems? Limno oceanog 40(2):386–392
Williamson CE, Metgar SL, Lovera PA, Moeller RE (1997) Solar ultraviolet radiation and the spawning habitat of yellow Perch, Perca flavescens. Ecol Appl 7(3):1017–1023. doi:10.1890/1051-0761(1997)007[1017:SURATS]2.0.CO;2
Winckler K, Fidhiany L (1996) Significant influence of UVA on the general metabolism in the growing Ciclid fish, Cichlasoma nigrofasciatum. J photochem photob B. Biology 33:131–135
Wu RSS, Lam PKS (1997) Glucose-6-phosphate dehydrogenase and lactate dehydrogenase in the green-lipped mussel (Perna viridis). Possible biomarker for hypoxia in the marine environment. Water Res 31:2797–2801
Yeh S, Wang W, Huang C, Hu M (2005) Pro-oxidative effect of β-carotene and the interaction with flavonoids on UVA-induced DNA strand breaks in mouse fibroblast C3H10T1/2 cells. J Nutr Biochem 16(12):729–735. doi:10.1016/j.jnutbio.2005.03.012
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mekkawy, I.A.A., Mahmoud, U.M., Osman, A.G. et al. Effects of ultraviolet A on the activity of two metabolic enzymes, DNA damage and lipid peroxidation during early developmental stages of the African catfish, Clarias gariepinus (Burchell, 1822). Fish Physiol Biochem 36, 605–626 (2010). https://doi.org/10.1007/s10695-009-9334-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-009-9334-6