Abstract
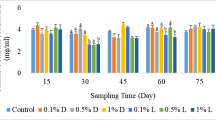
This study was conducted to evaluate the effects of dietary riboflavin on antioxidant defense in the juvenile grouper Epinephelus coioides. Graded levels of riboflavin (0.9, 1.6, 4.4, 6.7, 12.9 and 19.4 mg kg−1 dry diet) were fed to grouper juveniles (mean weight: 14.90 ± 0.46 g) for 12 weeks. Higher levels of liver thiobarbituric acid reactive substances (TBARS) content were observed in grouper fed low doses (0.9 and 1.6 mg kg−1 diet) of riboflavin. Both liver glutathione reductase (GR) activity and its activation coefficient (GR-AC) poorly responded to riboflavin deficiency. In addition, other indices of the glutathione-dependent defense system, including the activities of glutathione peroxidase (GSH-PX) and glutathione-S-transferase (GST), and the content of glutathione (GSH), were also non-significantly affected by dietary riboflavin levels. However, the activities of liver superoxide dismutase (SOD) and catalase (CAT) were significantly lower in fish fed 0.9 mg kg−1 diet, with a positive correlation between the different groups. In conclusion, the present study indicated that the juvenile grouper fed the riboflavin-unsupplemented diet was susceptible to lipid peroxidation (LPO), with lower SOD and CAT activities in the liver. However, the glutathione-dependent defense system of grouper was not affected by dietary riboflavin levels.


Similar content being viewed by others
Abbreviations
- CAT:
-
Catalase
- FAD:
-
Flavin adenine dinucleotide
- FMN:
-
Flavin mononucleotide
- GR:
-
Glutathione reductase
- GR-AC:
-
The activation coefficient of GR
- GSH:
-
Glutathione
- GSH-PX:
-
Glutathione peroxidase
- GSSG:
-
Glutathione disulfide
- GST:
-
Glutathione-S-transferase
- LPO:
-
Lipid peroxidation
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TBARS:
-
Thiobarbituric acid reactive substances
References
Adelekan DA, Thurnham DI (1998) Glutathione peroxidase (EC 111.1.9) and superoxide dismutase (EC 1.15.1.1) activities in riboflavin-deficient rats infected with Plasmodium berghei malaria. Br J Nutr 79:305–309. doi:10.1079/BJN19980048
Alvarez MJ, Lopez-Bote CJ, Diez A, Corraze G, Arzel J, Dias J, Kaushik SJ, Bautista SJ (1998) Dietary fish oil and digestible protein modify susceptibility to lipid peroxidation in the muscle of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax). Br J Nutr 80:281–289
Andersen F, Lygren B, Maage A, Waagbø R (1998) Interaction between two dietary levels of iron and two forms of ascorbic acid and the effect on growth, antioxidant status and some non-specific immune parameters in Atlantic salmon (Salmo salar) smolts. Aquaculture 161:437–451. doi:10.1016/S0044-8486(97)00291-3
AOAC(Association of Official Analytical Chemists) (1990) Official methods of analysis, no. 970.65, 15th edn. AOAC, Arlington, Va
Bates CJ (1991) Glutathione and reduced indices in rat lenses, liver and red cells during riboflavin deficiency and its correction. Exp Eye Res 53:123–130. doi:10.1016/0014-4835(91)90154-7
Bates CJ (1993) Riboflavin. Int J Vitam Nutr Res 63:274–277
Dabrowski K, Lee KJ, Guz L, Verlhac V, Gabaudan J (2004) Effects of dietary ascorbic acid on oxygen stress (hypoxia or hyperoxia), growth and tissue vitamin concentrations in juvenile rainbow trout (Oncorhynchus mykiss). Aquaculture 233:383–392. doi:10.1016/j.aquaculture.2003.09.047
Dutta P, Gee M, Rivlin RS, Pinto J (1988) Riboflavin deficiency and glutathione metabolism in rats: possible mechanisms underlying altered responses to hemolytic stimuli. J Nutr 118:1149–1157
Dutta P, Seirafi J, Halpin D, Pinto J, Rivlin R (1995) Acute ethanol exposure alters hepatic glutathione metabolism in riboflavin deficiency. Alcohol 12:43–47. doi:10.1016/0741-8329(94)00068-O
Fontagné S, Bazin D, Brèque J, Vachot C, Bernarde C, Rouault T, Bergot P (2006) Effects of dietary oxidized lipid and vitamin A on the early development and antioxidant status of Siberian sturgeon (Acipenser baeri) larvae. Aquaculture 257:400–411. doi:10.1016/j.aquaculture.2006.01.025
Garling DL, Wilson RP Jr (1977) Effect of dietary carbohydrate to lipid ratio on growth and body composition of fingerling channel catfish. Prog Fish-Cult 39:43–47. doi:10.1577/1548-8659(1977)39[43:EODCRO]2.0.CO;2
George S, Rilcy C, McEvoy J, Wright J (2000) Development of a fish in vitro cell culture model to investigate oxidative stress and its modulation by dietary vitamin E. Mar Environ Res 50:541–544. doi:10.1016/S0141-1136(00)00126-4
Halliwell B, Gutteridge JMC (1999) Free radicals in biology and medicine, 3rd edn. Oxford University Press, Oxford, pp 1–936
Hermes-Lima M (2004) Oxygen in biology and biochemistry: role of free radicals. In: Storey KB (ed) Functional metabolism regulation and adaptation. Wiley-Liss, Hoboken, pp 319–368
Hirano H, Hamajima S, Horiuchi S, Niitsu Y, Ono S (1983) Effects of B2-deficiency on lipoperoxide and its scavenging system in the rat lens. Int J Vitam Nutr Res 53:377–382
Huang JW, Tian LX, Du ZY, Yang HJ, Liu YJ (2005) Pyridoxine deficiency of grouper, Epinephelus coioides: physiological and biochemical alteration. Fish Physiol Biochem 31:331–337. doi:10.1007/s10695-005-2832-2
Huang JW, Tian LX, Du ZY, Yang HJ, Liu YJ (2007) Effects of dietary thiamin on the physiological status of the grouper Epinephelus coioides. Fish Physiol Biochem 33:167–172. doi:10.1007/s10695-007-9127-8
Kaplowitz N, Aw TK, Ookhtens M (1985) The regulation of hepatic glutathione. Annu Rev Pharmacol Toxicol 25:715–744. doi:10.1146/annurev.pa.25.040185.003435
Kodentsova VM, Vrzhesinskaia OA, Beketova NA, Pereverzeva OG, Kharitonchik LA, Lavrenťeva IB, Trofimenko AV, Spirichev VB (2003) The connection between vitamin and antioxidant status of the children with decreased hemoglobin level. Vopr Pitan 72:3–7
Kono Y, Fridovich I (1982) Superoxide radical inhibits catalase. J Biol Chem 257:5751–5754
Kucukbay Z, Yazlak H, Sahin N, Tuzcu M, Cakmak MN, Gurdogan F, Juturu V, Sahin K (2006) Zinc picolinate supplementation decreases oxidative stress in rainbow trout (Oncorhynchus mykiss). Aquaculture 257:465–469. doi:10.1016/j.aquaculture.2006.03.005
Lee SS, Ye JH, Jones DP, McCormick DB (1983) Correlation of H2O2 production and liver catalase during riboflavin deficiency and repletion in mammals. Biochem Biophys Res Commun 117:788–793. doi:10.1016/0006-291X(83)91666-2
Levin G, Cogan U, Levy Y, Mokady S (1990) Riboflavin deficiency and the function and fluidity of rat erythrocyte membranes. J Nutr 120:857–861
Lin YH, Shiau SY (2005) Dietary selenium requirements of juvenile grouper, Epinephelus malabaricus. Aquaculture 250:356–363. doi:10.1016/j.aquaculture.2005.03.022
Lopez-Bote CJ, Diez A, Corraze G, Arzel J, Alvarez MJ, Dias J, Kaushik SJ, Bautista JM (2001) Dietary protein source affects the susceptibility to lipid peroxidation of rainbow trout (Oncorhynchus mykiss) and sea bass (Dicentrarchus labrax) muscle. Anim Sci 73:443–449
Mackie AM, Mitchell AI (1985) Identification of gustatory feeding stimulants for fish-application in aquaculture. In: Cowey CB, Mackie AM, Bell JB (eds) Nutrition and feeding in fish. Academic Press, London, pp 177–189
Michiels C, Raes M, Toussaint O, Remacle J (1994) Importance of Se-glutathione peroxidase, catalase, and Cu/Zn-SOD for cell survival against oxidative stress. Free Radic Biol Med 17:235–248. doi:10.1016/0891-5849(94)90079-5
Mourente G, Díaz-Salvagoa E, Bell JG, Tocher DR (2002) Increased activities of hepatic antioxidant defence enzymes in juvenile gilthead sea bream (Sparus aurata L.) fed dietary oxidised oil: attenuation by dietary vitamin E. Aquaculture 214:343–361. doi:10.1016/S0044-8486(02)00064-9
Ohkawa H, Oishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358. doi:10.1016/0003-2697(79)90738-3
Okayasu T, Kameda K, Ono T, Imai Y (1977) Effects of dietary vitamin B-2 and vitamin E on the delta9-desaturase and catalase activities in the rat liver microsomes. Biochim Biophys Acta 489:397–402
Rueda-Jassoa R, Conceição LEC, Diasc J, De Coend W, Gomesc E, Reese JF, Soaresb F, Dinisb MT, Sorgeloos P (2004) Effect of dietary non-protein energy levels on condition and oxidative status of Senegalese sole (Solea senegalensis) juveniles. Aquaculture 231:417–433. doi:10.1016/S0044-8486(03)00537-4
Stéphan G, Guillaume J, Lamour F (1995) Lipid peroxidation in turbot (Scophthalmu maximus) tissue: effect of dietary vitamin E and dietary n-6 or n-3 polyunsaturated fatty acids. Aquaculture 130:251–268. doi:10.1016/0044-8486(94)00322-F
Taniguchi M, Hara T (1983) Effects of riboflavin and selenium deficiencies on glutathione and its relating enzyme activities with respect to lipid peroxide content of rat livers. J Nutr Sci Vitaminol (Tokyo) 29:283–292
Toyosaki T (1992) Antioxidant effect of riboflavin in enzymatic lipid peroxidation. J Am Chem Soc 40:1727–1730
Toyosaki T, Mineshita T (1988) Antioxidant effects of protein-bound riboflavin and free riboflavin. J Food Sci 6:1851–1853. doi:10.1111/j.1365-2621.1988.tb07859.x
Trenzado C, Hidalgo MC, García-Gallego M, Morales AE, Furné M, Domezain A, Domezain J, Sanz A (2006) Antioxidant enzymes and lipid peroxidation in sturgeon Acipense naccarii and trout Oncorhynchu mykiss: A comparative study. Aquaculture 254:758–767. doi:10.1016/j.aquaculture.2005.11.020
Tumkiratiwong P, Tungtrongchitr R, Migasena P, Pongpaew P, Rojekittikhun W, Vudhivai N, Tungtrongchitr A, Phonrat B, Nuamtanong S (2003) Antioxidant enzyme levels in the erythrocytes of riboflavin-deficient and Trichinella spiralis-infected rats. Southeast Asian J Trop Med Public Health 34:480–485
Wilhelm Filho D, Giulivi C, Boveris A (1993) Antioxidant defences in marine fish—I. Teleosts. Comp Biochem Physiol 106C:409–413
Winston GW, Di Giulio RT (1991) Prooxidant and antioxidant mechanism in aquatic organisms. Aquat Toxicol 19:137–161. doi:10.1016/0166-445X(91)90033-6
Woodward B (1983) Sensitivity of hepatic D-amino acid oxidase and glutathione reductase to the riboflavin status of the rainbow trout (Salm gairdneri). Aquaculture 34:193–201. doi:10.1016/0044-8486(83)90202-8
Wu WK, Mak CH, Ko RC (2006) Cloning and characterization of the Cu/Zn superoxide dismutase of Trichinella pseudospiralis. Parasitol Res 98:281–287. doi:10.1007/s00436-005-0056-0
Zanetti G, Beretta C, Malandra D (1986) Properties of rabbit liver glutathione reductase reconstituted with FAD analogs. Arch Biochem Biophys 244:831–837. doi:10.1016/0003-9861(86)90652-1
Acknowledgements
This project was supported by the Key Technologies R&D Program during the 11th 5-year plan, China (grant no. 2006BAD03B03).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, J., Tian, L., Wu, X. et al. Effects of dietary riboflavin levels on antioxidant defense of the juvenile grouper Epinephelus coioides . Fish Physiol Biochem 36, 55–62 (2010). https://doi.org/10.1007/s10695-008-9279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10695-008-9279-1