Abstract
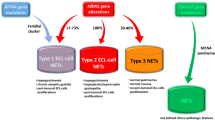
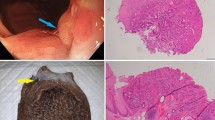
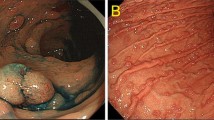
Lower gastrointestinal (GI) neuroendocrine neoplasms (NENs) of the colon and rectum are uncommon and not traditionally associated with hereditary GI cancer syndromes. However, with widespread implementation of colorectal cancer screening programs, lower GI NENs are being identified with increasing frequency. We report the first case series of six patients with lower GI NENs who were diagnosed with hereditary GI cancer syndromes by germline testing. Two patients presented with poorly differentiated rectal neuroendocrine carcinoma (NECs) with colonic polyposis and were found to have Familial Adenomatous Polyposis and MYH-Associated Polyposis, respectively. Three patients with colorectal NENs (one well differentiated neuroendocrine tumor, NET, and two NECs), all of which displayed abnormal immunohistochemistry for mismatch repair proteins, were diagnosed with Lynch syndrome. One patient with a goblet cell carcinoid was diagnosed with CHEK2 mutations. All patients met genetic testing guidelines and the diagnosis was made utilizing next generation sequencing gene panel tests. Lower GI NETs should therefore be considered a potential hereditary GI cancer syndrome-associated malignancy in patients who otherwise meet criteria for genetic evaluation.

Similar content being viewed by others
References
Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW, American College of G (2015) ACG clinical guideline: genetic testing and management of hereditary gastrointestinal cancer syndromes. Am J Gastroenterol 110:223–262
Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64:9–29
Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A, Evans DB (2008) One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the united states. J Clin Oncol 26:3063–3072
Tsikitis VL, Wertheim BC, Guerrero MA (2012) Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: A seer analysis. J Cancer 3:292–230
Basuroy R, Haji A, Ramage JK, Quaglia A, Srirajaskanthan R (2016) Review article: The investigation and management of rectal neuroendocrine tumours. Aliment Pharmacol Ther 44:332–345
Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S (2010) The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 39:707–712
Kidd M, Modlin I, Oberg K (2016) Towards a new classification of gastroenteropancreatic neuroendocrine neoplasms. Nat Rev Clin Oncol 13:691–705
Bernick PE, Klimstra DS, Shia J, Minsky B, Saltz L, Shi W, Thaler H, Guillem J, Paty P, Cohen AM, Wong WD (2004) Neuroendocrine carcinomas of the colon and rectum. Dis Colon Rectum 47:163–169
Hall MJ, Forman AD, Pilarski R, Wiesner G, Giri VN (2014) Gene panel testing for inherited cancer risk. J Natl Compr Canc Netw 12:1339–1346
Hansen MF, Johansen J, Sylvander AE, Bjornevoll I, Talseth-Palmer BA, Lavik LA, Xavier A, Engebretsen LF, Scott RJ, Drablos F, Sjursen W (2017) Use of multigene-panel identifies pathogenic variants in several crc-predisposing genes in patients previously tested for lynch syndrome. Clin Genet. doi:10.1111/cge.12994
Pearlman R, Frankel WL, Swanson B et al (2016) Prevalence and spectrum of germline cancer susceptibility gene mutations among patients with early-onset colorectal cancer. JAMA Oncol. doi:10.1001/jamaoncol.2016.5194
Yadav S, Reeves A, Campian S et al (2016) Outcomes of retesting brca negative patients using multigene panels. Fam Cancer. doi:10.1007/s10689-016-9956-7
LaDuca H, Stuenkel AJ, Dolinsky JS, Keiles S, Tandy S, Pesaran T, Chen E, Gau CL, Palmaer E, Shoaepour K, Shah D, Speare V, Gandomi S, Chao E (2014) Utilization of multigene panels in hereditary cancer predisposition testing: analysis of more than 2,000 patients. Genet Med 16:830–837
Mauer CB, Pirzadeh-Miller SM, Robinson LD, Euhus DM (2014) The integration of next-generation sequencing panels in the clinical cancer genetics practice: an institutional experience. Genet Med 16:407–412
Kurian AW, Hare EE, Mills MA, Kingham KE, McPherson L, Whittemore AS, McGuire V, Ladabaum U, Kobayashi Y, Lincoln SE, Cargill M, Ford JM (2014) Clinical evaluation of a multiple-gene sequencing panel for hereditary cancer risk assessment. J Clin Oncol 32:2001–2009
Yousef I, Siyam F, Layfield L, Freter C, Sowers JR (2014) Cervical neuroendocrine tumor in a young female with lynch syndrome. Neuro Endocrinol Lett 35:89–94
Sorscher S, Saroya B (2013) A molecularly confirmed neuroendocrine tumor resulting from lynch syndrome. J Gastrointest Oncol 4:95–96
Miquel C, Sabourin JC, Elias D, Grandjouan S, Viguier J, Ducreux M, Duvillard P, Praz F (2004) An appendix carcinoid tumor in a patient with hereditary nonpolyposis colorectal cancer. Hum Pathol 35:1564–1567
Estrella JS, Taggart MW, Rashid A, Abraham SC (2014) Low-grade neuroendocrine tumors arising in intestinal adenomas: evidence for alterations in the adenomatous polyposis coli/beta-catenin pathway. Hum Pathol 45:2051–2058
July LV, Northcott KA, Yoshida EM, Carr DM, Owen DA (1999) Coexisting carcinoid tumors in familial adenomatous polyposis-associated upper intestinal adenomas. Am J Gastroenterol 94:1010–1091
Giardiello FM, Allen JI, Axilbund JE et al (2014) Guidelines on genetic evaluation and management of lynch syndrome: a consensus statement by the us multi-society task force on colorectal cancer. Am J Gastroenterol 109:1159–1179
Stelow EB, Moskaluk CA, Mills SE (2006) The mismatch repair protein status of colorectal small cell neuroendocrine carcinomas. Am J Surg Pathol 30:1401–1404
Kloppel G, Rindi G, Anlauf M, Perren A, Komminoth P (2007) Site-specific biology and pathology of gastroenteropancreatic neuroendocrine tumors. Virchows Arch 451(Suppl 1):S9–S27
Oberg K (2013) The genetics of neuroendocrine tumors. Semin Oncol 40:37–44
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare they have no conflict of intreset.
Informed consent
Obtained from patients.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kidambi, T.D., Pedley, C., Blanco, A. et al. Lower gastrointestinal neuroendocrine neoplasms associated with hereditary cancer syndromes: a case series. Familial Cancer 16, 537–543 (2017). https://doi.org/10.1007/s10689-017-9979-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10689-017-9979-8