Abstract
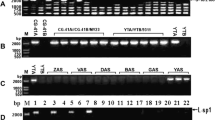
A novel and stable cytoplasmic male sterility CMS line of tuber mustard has been bred by subsequent backcrosses for 10 years. Two specific markers atpA and orf220 were cloned and partially characterized in our previous study (Zhang et al. 2003). In this study, two new molecular markers, orf256 and orf305/orf324, have been isolated and identified. The orf256 gene size was found to be 825 bp in CMS line and a 1,357 bp in its maintainer line. Sequence analysis indicated that the orf256 gene was an entire coding sequence and downstream of the cox1 gene. Interestingly, the 906 bp fragment, which contains part of the sequence of orf222, nad5 and orf139 genes, was found to be inserted from the 451st bp of 5′-flank of the 1,357 bp fragment. In the same way, the orf324 gene was isolated from CMS line and orf305 gene from its maintainer line. Both of them are entire coding sequences, upstream from nad3 and rps12 gene, and co-transcribed with the nad3 and rps12 genes. In addition, two molecular markers, orf256 and orf324/orf305, have been successfully converted into the SCAR markers. Subsequently, ORF256, ORF324, ORF305 protein and ORF256-M-431 fragment are predicated to contain signal peptide sequences, and ORF220 was predicated to contain signal anchor sequence. RFLP analysis results revealed that all of the molecular markers exhibited polymorphisms. Northern blot analysis indicated that the expression level of these genes in CMS line is higher than that of the maintainer line. In the mass, all of these genes are expressed lower in the leaf than that of floral organs between the CMS line and its maintainer line. The difference in expression pattern of different mitochondrial specific marker genes suggests that the abundance of mitochondrial proteins is differentially regulated in the organ/tissue development in tuber mustard. Results of this study also provide some novel and useful clues to explore the biological function of these specific marker genes in the tuber mustard.





Similar content being viewed by others
Abbreviations
- CMS:
-
Cytoplasmic male sterility
- PCR:
-
Polymerase chain reaction
- RT-PCR:
-
Reverse transcription polymerase chain reaction
- ORF:
-
Open reading frame
- RNAi:
-
RNA interference
- RFLP:
-
Restriction fragment length polymorphism
- SCAR:
-
Sequence characterized amplified region
References
Akagi H, Nakamura A, Sawada R, Oka M, Fujimura T (1995) Genetic diagnosis of cytoplasmic male-sterile cybrid plants of rice. Theor Appl Genet 90:948–951. doi:10.1007/BF00222907
Aldrich J, Cullis CA (1993) RAPD analysis in flax: optimization of yield and reproducibility using KlenTaq 1 DNA polymerase, Chelex 100, and gel purification of genomic DNA. Plant Mol Biol Rep 11:128–141. doi:10.1007/BF02670471
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: signal P 3.0. J Mol Biol 340:783–795. doi:10.1016/j.jmb.2004.05.028
Brown GG, Mona D (1998) Molecular analysis of Brassica CMS and its application to hybrid seed production. Acta Hortic 459:265–274
Chen ZJ, Zhang MF, Wang BL (1995) A study on fertility and agronomic characters of CMS lines for tuber mustard. Acta Hortic Sin 22:40–45
Handa H (2003) The complete nucleotide sequence and RNA editing content of the mitochondrial genome of rapeseed (Brassica napus L.): comparative analysis of the mitochondrial genome of rapeseed and Arabidopsis thaliana. Nucleic Acids Res 31:5907–5916. doi:10.1093/nar/gkg795
Hanson M, Bentolila S (2004) Interactions of mitochondrial and nuclear gene that affect male gametophyte development. Plant Cell 16(suppl):S154–S169. doi:10.1105/tpc.015966
Hanson MR, Wilson RK, Bentolila S, Kohler RH, Chen HC (1999) Mitochondrial gene organization and expression in petunia male fertile and sterile plants. J Hered 90:362–368. doi:10.1093/jhered/90.3.362
Harold T, Albert B, Jan DH, Sierd B, Jan MD (2000) Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiol Mol Biol Rev 9:515–547
Hirata Y, Motegi T, Takeda Y, Morikawa K (2001) Induction of cytoplasmic male sterility in the seed progeny derived from artificial-synthesized interspecific chimera in Brassica. Euphytica 117:143–149. doi:10.1023/A:1004068520579
Kubo T, Nishizawa S, Mikami T (1999) Alterations in organization and transcription of the mitochondrial genome of cytoplasmic male sterile sugar beet (Beta vulgaris L.). Mol Gen Genet 262:283–290. doi:10.1007/s004380051085
Liu PY (1996) Chinese mustard. In: Liu PY (ed) Origin, evolution, classification and distribution of Chinese mustard. China Agriculture Press, Beijing, pp 1–26
Liu JH, Landgren M, Glimelius K (1996) Transfer of the Brassica tournefortii cytoplasm to B. napus for the production of cytoplasmic male sterile B. napus. Physiol Plant 96:123–129. doi:10.1111/j.1399-3054.1996.tb00192.x
Liu ZS, Guan CY, Chen SY (2001) Application and research on mechanism of plant male sterility. Press of Chinese Agriculture, Beijing, pp 1–316
Makaroff CA, Aepl IJ, Palmer JD (1989) The atp6 coding region has been disrupted and a novel reading frame generated in the mitochondria genome of cytoplasm male-sterile radish. J Biol Chem 264:11706–11713
Makaroff CA, Aepl IJ, Palmer JD (1990) Characterization of radish mitochondrial atpA: influence of nuclear background on transcription of atpA-associated sequences and relationship with male sterility. Plant Mol Biol 15:735–746. doi:10.1007/BF00016123
Makaroff CA, Aepl IJ, Palmer JD (1991) The role of coxI associated repeated sequence in the plant mitochondrial DNA rearrangement and radish cytoplasmic male-sterility. Curr Genet 19:183–190. doi:10.1007/BF00336485
Mihara K (2000) Targeting and insertion of nuclear-encoded preproteins into the mitochondrial outer membrane. Bioessays 22:364–371. doi:10.1002/(SICI)1521-1878(200004)22:4<364::AID-BIES6>3.0.CO;2-N
Mizumoto K, Murai K, Nakamura C, Takumi S (2004) Preferential expression of a HLP homolog encoding a mitochondrial L14 ribosomal protein in stamens of common wheat. Gene 343:281–289. doi:10.1016/j.gene.2004.09.005
Motegi T, Nou SI, Zhou JM, Kanno A, Kameya T, Hirata Y (2003) Obtaining an Ogura-type CMS line from asymmetrical protoplast fusion between cabbage (fertile) and radish (fertile). Euphytica 129:319–323. doi:10.1023/A:1022284803689
Nahm SH, Lee HJ, Lee SW, Joo GY, Harn CH, Yang SG et al (2005) Development of a molecular marker specific to a novel CMS line in radish (Raphanus sativus L.). Theor Appl Genet 111:1191–1200. doi:10.1007/s00122-005-0052-x
Notsu Y, Masood S, Nishikawa T, Kubo N, Akiduki G, Nakazono M et al (2002) The complete sequence of the rice (Oryza sativa L.) mitochondrial genome: frequent DNA sequence acquisition and loss during the evolution of flowering plants. Mol Genet Genomics 268:434–445. doi:10.1007/s00438-002-0767-1
Ogihara Y, Yamazaki Y, Murai K, Kanno A, Terachi T, Shiina T et al (2005) Structural dynamics of cereal mitochondrial genomes as revealed by complete nucleotide sequencing of the wheat mitochondrial genome. Nucleic Acids Res 33:6235–6250. doi:10.1093/nar/gki925
Okada K, Ohara K, Yazaki K, Nozaki K, Uchida N, Kawamukai M et al (2004) The AtPPT1 gene encoding 4-hydroxybenzoate polyprenyl diphosphate transferase in ubiquinone biosynthesis is required for embryo development in Arabidopsis thaliana. Plant Mol Biol 57:567–577. doi:10.1007/s11103-004-1298-4
Rankin CT, Cutright MT, Makaroff CA (1996) Characterization of the radish mitochondrial nad3/rps12 locus: analysis of recombination repeats and RNA editing. Curr Genet 29:564–571. doi:10.1007/BF02426961
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York, pp 343–388
Schenable PS, Wise RP (1998) The molecular basis of cytoplasmic male sterility and fertility restoration. Trends Plant Sci 3:175–180. doi:10.1016/S1360-1385(98)01235-7
Singh M, Brown GG (1993) Characterization of expression of a mitochondrial gene region associated with the Brassica ‘Polima’ CMS: developmental influences. Curr Genet 24:316–322. doi:10.1007/BF00336783
Sjöling S, Glaser E (1998) Mitochondrial targeting peptide in plants. Trends Plant Sci 3:136–140. doi:10.1016/S1360-1385(98)01212-6
Song JS, Hedgcoth C (1994a) Influence of nuclear background on transcription of a chimeric gene (orf256) and coxI in fertile and cytoplasmic male sterile wheats. Genome 37:203–209. doi:10.1139/g94-028
Song JS, Hedgcoth C (1994b) A chimeric gene (orf256) is expressed as protein only in cytoplasmic male-sterile lines of wheat. Plant Mol Biol 26:535–539. doi:10.1007/BF00039566
Sugiyama Y, Watase Y, Nagase M, Makita N, Yagura S, Hirai A, Sugiura M (2005) The complete nucleotide sequence and multipartite organization of the tobacco mitochondrial genome: comparative analysis of mitochondrial genomes in higher plants. Mol Gen Genomics 272:603–615
Triboush SO, Danilenko NG, Davydenko OG (1998) A method for isolation of chloroplast DNA and mitochondrial DNA from sunflower. Plant Mol Biol Rep 16:183–189. doi:10.1023/A:1007487806583
Unseld M, Marienfeld JR, Brandt P, Brennicke A (1997) The mitochondrial genome of Arabidopsis thaliana contains 57 genes in 366, 924 nucleotides. Nat Genet 15:57–61. doi:10.1038/ng0197-57
Yamasaki S, Konno N, Kishitani S (2004) Overexpression of mitochondrial genes is caused by interactions between the nucleus of Brassica rapa and the cytoplasm of Diplotaxis muralis in the leaves of alloplasmic lines of B. rapa. J Plant Res 117:339–344. doi:10.1007/s10265-004-0162-6
Young EG, Hanson MR (1987) A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell 50:41–49. doi:10.1016/0092-8674(87)90660-X
Zhang XP, Glaser E (2002) Interaction of plant mitochondrial and chloroplast signal peptides with the Hsp70 molecular chaperone. Trends Plant Sci 7:14–21. doi:10.1016/S1360-1385(01)02180-X
Zhang MF, Chen LP, Wang BL, Yang JH, Chen ZJ, Hirata Y (2003) Characterization of atpA and orf220 genes distinctively present in a cytoplasmic male-sterile line of tuber mustard. J Hortic Sci Biotechnol 78:837–841
Acknowledgments
This research was partially supported by the Ningbo Science Foundation of China (2004A410018). The authors gratefully acknowledge Dr. Gergely Gulyas for reading and criticizing this manuscript and Dr. Gen Hattori for his helpful advice.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, Xl., Xiao, Qb., Cao, Js. et al. Development of two new molecular markers specific to cytoplasmic male sterility in tuber mustard (Brassica juncea var. tumida Tsen et Lee). Euphytica 166, 367–378 (2009). https://doi.org/10.1007/s10681-008-9817-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10681-008-9817-z