Abstract
Eutrophication impairs lake ecosystems at a global scale. In this context, as benthic microalgae are well-established warnings for a large range of stressors, particularly nutrient enrichment, the Water Framework Directive required the development of diatom-based methods to monitor lake eutrophication. Here, we present the diatom-based index we developed for French lakes, named IBDL (Indice Biologique Diatomées en Lacs). Data were collected in 93 lakes from 2015 to 2020. A challenge arose from the discontinuous pressure gradient of our dataset, especially the low number of nutrient-impacted lakes. To analyze the data we opted for the so-called “Threshold Indicator Taxa ANalysis” method, which makes it possible to determine a list of “alert taxa.” We obtained a multimetric index based on specific pressure gradients (Kjeldahl nitrogen, suspended matter, biological oxygen demand, and total phosphorous). Considering the European intercalibration process, the very good correlation between IBDL and the common metric (R2 from 0.52 to 0.87 according to the lake alkalinity type) makes us very confident in our ability to match future IBDL quality thresholds with European standards. The IBDL proved at last to be particularly relevant as it has a twofold interest: an excellent relationship with total phosphorus (R2 from 0.63 to 0.83 according to the lake alkalinity type) and a possible application to any lake metatype. Its complementarity with macrophyte-based indices moreover justifies the use of at least two primary producer components for lake ecological status classification.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Eutrophication is one of the most frequent consequences of human pressure on lake ecosystems at a global scale (Stenger-Kovács et al., 2007). Primary producers are directly impacted since they are the base of the aquatic food web (Brauer et al., 2012). As the ability of species to compete differs according to nutrient availability, nutrient enrichment results in significant changes in community structure and function (Birk et al., 2012). For this reason, scientists and policymakers developed indices based on primary producer attributes to monitor eutrophication (Stevenson, 2014). In the early 2000s, the Water Framework Directive (European Union, 2000) required all EU member states to implement bioassessment methods based, among other aspects, on the biological quality of “macrophytes and phytobenthos” to assess lake ecological status. This led to the development of numerous methods at the European level.
Poikane et al. (2016) reviewed this panel of methods and observed that countries generally developed separate assessment tools for macrophytes and phytobenthos, and that most of them considered diatoms, which are unicellular microalgae, to be a good proxy for phytobenthos. Diatoms are indeed early and well-established warnings for a large range of stressors, particularly nutrient enrichment (Stevenson, 2014). As a first step, indices originally dedicated to rivers were applied to lakes by the majority of member states (Kelly et al., 2014b), considering that many processes influencing diatom assemblages were comparable between lakeshores and shallow rivers (Cantonati & Lowe, 2014).
In some rare cases, diatom-based indices were developed specifically for lakes, based on species composition and abundance as for rivers (Bennion et al., 2014; Poikane et al., 2016). Diatoms from mud and silts were generally not considered, as they would respond to pore-water chemistry rather than water quality. The recommended sampling substrate varied according to authors, from macrophytes to cobbles or even artificial substrates when no natural substrates are found in all water bodies (King et al., 2006).
To harmonize the different national approaches, a European intercalibration exercise was performed, involving eleven member states (Kelly et al., 2014b). France participated in this exercise with the Biological Diatom Index (BDI, Coste et al., 2009), routinely used to assess river ecological status. Although previous results tended to suggest there was a good correlation between BDI and the environmental pressure gradients, at least in shallow lakes (Cellamare et al., 2012), this intercalibration exercise revealed a poor correlation between BDI values and total phosphorous across France (Kelly et al., 2014b). This was explained by the absence of many lake taxa from the list of key species used to calculate the BDI, resulting in an overall poor relevance of the final status assessment.
The aim of the present study was, therefore, to develop a new diatom-based index for lakes in metropolitan France: the IBDL (Indice Biologique Diatomées en Lac: Diatom Biological Index for Lakes). To collect the necessary data, we proposed a method (Morin et al., 2010) consistent with a potential subsequent combination of this index with the existing French macrophyte index IBML (Indice Biologique Macrophytique en Lac: Macrophyte Biological Index for Lakes, Boutry et al., 2015). We detail here how diatom data were sampled and analyzed and how we developed the IBDL. Finally, we discuss the relevance of this new index, comparing the results obtained with index scores based on macrophytes, and assessing its ability to reveal environmental gradients.
Materials and methods
Data collection
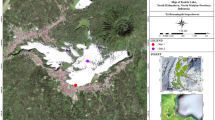
Samples were collected from 93 French lakes during the summer period, between 2015 and 2020 as part of national assessment surveys, according to Morin et al. (2010) (Fig. 1 and S1 Table 1). The lakes were classified into three metatypes based on alkalinity, according to the European intercalibration exercise previously performed (Kelly et al., 2014b): low alkalinity (LA, alkalinity ≤ 0.2 meq.l−1), medium alkalinity (MA, 0.2 meq.l−1 < alkalinity < 1 meq.l−1), and high alkalinity (HA, alkalinity ≥ 1 meq.l−1). Diatoms were collected from both mineral substrates and lakeshore macrophyte surfaces in observation units (OUs), whose number and location varied according to the lake surface area and the riparian zone types. Such units are defined in the French macrophyte sampling protocol for lakes NF T90-328 (AFNOR, 2022).
Study sites, number of surveys per site, and lake alkalinity classes (LA, low alkalinity; MA, medium alkalinity; HA, high alkalinity) (Kelly et al., 2014a)
Biological data
Samples from hard mineral substrates were taken from at least five boulders or cobbles selected at random for each OU. The total surface area sampled was equivalent to 100 cm2, as defined in the NF T90-354 standard (AFNOR, 2016). Selected substrates had to be submerged within the euphotic zone at a maximum depth of 0.5 m.
Samples performed on macrophytes were taken from helophytes (mainly Phragmites australis (Cav.) Trin. ex Steud.). Green stem segments submerged for at least 4 to 6 weeks were collected from a minimum of 5 macrophytes chosen at random. These stem segments had to be located at a maximum depth of 0.2 m.
Diatoms were sampled from both substrates according to the NF T90-354 protocol, in line with the European standards (EN 13946; CEN, 2003). Cells were identified at 1000× magnification by examining permanent slides of cleaned diatom frustules (400 valves per slide) using, among others, Krammer and Lange-Bertalot (1986–1991) and Lange-Bertalot (1995–2015, 2000–2013). Taxonomic homogenization was performed with Omnidia 6 software (Lecointe et al., 1993).
All OUs from a single lake were sampled within a maximum of 21 days. Diatom counts had to include at least 350 cells per slide, with more than 50% of the diatom cells determined at the species level, to comply with the NF T90-354 requirements.
Physico-chemical data
Parameter values were determined in summer in the euphotic layer at the deepest point of each lake, according to European standards. Data were obtained from national surveillance monitoring programs. Water quality analysis was not systematically performed each year: in a few cases (10% of the samples), the most recent physicochemical data available were collected the year after or before the diatom samples. The following parameters were recorded: biological oxygen demand (BOD5, mg.l−1), oxygen (O2, mg.l−1), oxygen saturation (% O2), conductivity (Cond, µs.cm2), Kjeldahl nitrogen (NKJ, mg.l−1), ammonium (NH4, mg.l−1), nitrates (NO3, mg.l−1), nitrites (NO2, mg.l−1), orthophosphates (PO4, mg.l−1), total phosphorous (Pt, mg.l−1), and suspended particles (SP, mg.l−1).
Data analysis and index settlement
All analyses were performed with R version 4.1.2 (2021–11-01) (R Core Team, 2021) (Platform: x86_64-pc-linux-gnu (64-bit), Running under: Ubuntu 22.04.1 LTS).
Considering that the final dataset revealed a discontinuous trophic gradient, we opted for the so-called Threshold Indicator Taxa ANalysis method (TITAN2 package, Baker et al., 2020), which, based on bootstrapping and permutations, makes it possible to determine a list of “alert taxa.” The presence and/or increasing abundance of alert taxa reveals the existence of anthropogenic pressures. TITAN replaces the community‐level response along a composite gradient with taxon‐specific responses toward single-environmental variables (Dufrêne & Legendre, 1997). Negative and positive responses are distinguished, and cumulative decreasing or increasing responses in the community are tracked. This method is particularly suitable for setting up multimetric indices.
A three-step procedure was necessary to build our biological diatom index for lakes (IBDL): identification of alert taxa, choice of relevant metrics, and aggregation of these metrics to obtain the final index score.
Identification of alert taxa
For the next part of the analysis, we set an occurrence threshold ≥ 3 for taxa to be included in the index calculation (the so-called index taxa).
TITAN combines change-point analysis (nCPA; King & Richardson, 2003) and indicator species analysis (IndVal, Dufrêne et al., 1997). Basically, the change-point analysis compares within-group vs. between-group dissimilarity to detect shifts in community structure along the environmental variable considered (for further details concerning this method, see Baker and King (2010)). Indicator species analysis then identifies the strength of association between any particular taxon and this sample grouping. At the end of the process, two IndVal scores are calculated for a single taxon in a two-group classification. The algorithm finally classifies taxa into three different categories: Z+ taxa, showing a significant increase in abundance along the increasing environmental gradient; Z− taxa, showing a significant decrease along this gradient; and indifferent taxa, with no significant trend.
Alert taxa were defined as Z+ or Z− taxa whose shift thresholds were greater or lesser than the community shift threshold.
Building metrics and selecting the relevant ones
For each environmental variable, a metric was calculated at the OU scale according to (1)
where Alerttaxa is the number of alert taxa and Indextaxa is the number of index taxa in the sample.
The metric value is bounded between 0 and 1. The lowest value (0) corresponds to a species list entirely composed of alert taxa (determined for the environmental variable considered).
To build our index, we then selected the most relevant metrics, i.e., those with the best relationship with the environmental parameter considered. We used Pearson’s correlation coefficients to measure this statistical association and only kept metrics showing a Pearson’s coefficient over 0.6. Metrics should significantly increase with impairment, significantly decrease with impairment, or show no particular pattern. We obtained the response patterns of the different metrics by transforming raw values into normalized deviations (standardized effect size: SES, Gotelli & McCabe, 2002; Mondy et al., 2012) (2). SES values made it possible to obtain a single response pattern for a metric whatever the lake metatype and substrate type considered.
where MetricM is the observed value of the metric, and Mgroup and sdgroup are the mean and standard deviation, respectively, of the metric value for a given group of samples (i.e., substrate type × lake alkalinity metatype) (values of Mgroup and sdgroup are given in Table 1 S2).
The next step consisted of the normalization of SES values (SESnorM) to make comparable metric variation ranges (3):
where SESM is the observed value of SES for a given metric, Min its minimum value, and Max its maximum value in the whole dataset (values of Min and Max are given in Table 2 S2).
We further transformed metric values from normalized SES into the ecological quality ratio (EQR) (4), i.e., the ratio between the observed value of a metric (SESnorM) and its expected value under reference conditions, for any lake metatype and any substrate (SESnorMref, values given in Table 3 S2).. National reference conditions were set based on lakes characterized by very low or negligible anthropic pressure. This selection was checked according to the land use criteria applied during the initial lake intercalibration exercise (Kelly et al., 2014a). Lakes were deemed to be in reference condition if showing < 0.4% artificial land use and < 20% agriculture within the catchment area.
Finally, for each metric, we performed a Wilcoxon test to detect the potential influence of substrate type on the EQR values obtained at the OU scale.
Aggregating metric values to obtain the final IBDL score
The final index score was obtained at the OU scale by averaging the selected metric values, expressed in EQR.
For a score calculated for both mineral and macrophyte substrates, the lowest value was considered the final score.
Each OU belongs to one of the four riparian zone types, as required in the NF T90-328 standard (AFNOR, 2022). These types were defined from the vegetation composition and/or anthropogenic alterations of the lakeshore. The percentage of each riparian zone type was estimated in situ, on the whole lake perimeter, during the sampling surveys. The final index score for the whole lake was derived from a weighted average of the ScoreOU (5), taking into account the percentage of the lake perimeter each OU represented in terms of riparian zone type (Pctype).
Finally, the resulting IBDL scores varied between 0 (worst water quality) and 1. Relationships between IBDL scores and the different environmental variables considered were tested a posteriori with simple linear regressions (R “mass” package, Venables & Ripley, 2002).
Comparing IBDL and IBML scores
We compared IBDL and IBML scores, based, respectively, on diatom and macrophyte communities to evaluate their complementarity or redundancy. IBML scores were computed with the online application https://seee.eaufrance.fr/api/indicateurs/IBML/1.0.1 and the “httr” package (Wickham, 2022).
We built a multiple linear regression model (“mass” package) to test which index correlated best with Pt values: IBML, IBDL, or a combination of both (mean value).
Preparing intercalibration
Considering a future intercalibration exercise, we analyzed the relationships between IBDL scores and Pt for each lake metatype. A good correlation of the candidate metric with Pt constitutes a key criterion for considering the index ready for integration into the intercalibration process (Kelly et al., 2014b).
We also plotted IBDL against CM scores (intercalibration common metric, i.e., the trophic index developed by Rott et al., 1998) to check their compliance. The CM was calculated with Omnidia 6 software.
Results
Our data revealed discontinuous pressure gradients (Table 1), with a clear lack of impacted conditions and an over-representation of lakes characterized by low eutrophication levels.
sd, standard deviation; p25, 25th percentile; p75, 75th percentile.
Biotic and abiotic data were obtained for 958 samples. Considering the data validation criteria, 99% of the samples were included in the analysis. Sixty-eight, 202, and 402 OUs were, respectively, sampled on LA, MA, and HA lakes (unknown alkalinity type for 8 lakes). Table 2 S1 specifies the substrates sampled for each alkalinity type. Data from both substrate types were available for 552 OUs. Seven hundred eighty taxa were recorded, 8% of which were identified to the genus level. One hundred and twenty-one alert taxa were determined out of 590 index taxa (S3).
We obtained the following Pearson test values for the different metrics at the OU scale: R = −0.715 for the metric based on the parameter NKJ, R = −0.754 for BOD5, R = −0.688 for Pt, R = −0.666 for SP, R = −0.553 for PO4, R = −0.329 for conductivity, R = −0.174 for O2, R = −0.265 for NO2, and R = −0.204 for %O2. Considering the selection rule proposed (|R|> 0.6), only the metrics based on NKJ, BOD5, Pt, and SP were considered to build the IBDL.
Metric values (in EQR) calculated from the lists of taxa sampled on mineral substrates and macrophytes for a single OU did not differ significantly (p-value = 0.65).
IBDL scores at the lake level were calculated from the selected metrics following the aggregation rules proposed. The scores obtained were distributed as given in Fig. 2. IBDL could not be calculated for 20% of the samples due to incomplete floristic data.
The relationships between IBDL scores and the different environmental variables considered were very good (Fig. 3) in both high-alkalinity and medium-alkalinity lakes. IBDL scores showed high correlations with these variables, particularly Pt, in both high alkalinity (R2 = 0.63, p = 1.8e−15) and medium alkalinity lakes (R2 = 0.83, p = 8.3e−11). Note that data from low alkalinity lakes were too scarce to perform such correlations.
IBDL scores were also strongly associated with CM scores (R2 = 0.52 and p = 2.2e−16 for high-alkalinity lakes; R2 = 0.87 and p = 1.8 e−7 for medium-alkalinity lakes) (Fig. 4).
IBDL scores showed a better correlation with Pt (AIC = −171.44) than did IBML (AIC = −129.25) or a combination of both indices (AIC = −169.44). Nevertheless, IBDL tended to be generally less stringent than IBML (in 18 out of 22 samples), especially for scores higher than 0.8 (clearly dominant here). Figure 5 presents the difference between IBDL and IBML scores according to IBDL scores.
Discussion
As required by the WFD, we developed a diatom index for the assessment of the ecological status of French lakes. We obtained very good correlations between IBDL and key environmental variables. One major challenge arose from the discontinuous pressure gradient of our dataset, especially the low available number of nutrient-impacted lakes.
The scarcity of impacted lakes in the datasets used to build diatom indices is not rare and has already been pointed out by some authors (Bennion et al., 2014). This lack makes it impossible to capture the entire trophic gradient or to build reliable species’ ecological profiles. However, the majority of existing indices are calculated as an abundance-weighted average of the ecological profiles of every taxon from a sample, according to the Zelinka and Marvan formula (Zelinka & Marvan, 1961). This method is far from optimal for datasets showing discontinuous or very specific environmental conditions (Carayon et al., 2020). In such cases, the identification of alert taxa seems more appropriate than considering diatom communities as a whole. This has made the TITAN algorithm increasingly popular for detecting specific taxa providing reliable signals of a specific stress (Carayon et al., 2020; Costas et al., 2018; Gieswein et al., 2019; Gonzalez-Paz et al., 2020; Khamis et al., 2014).
Using this method, we built a multimetric index based on different pressure gradients (NKJ, SP, BOD5, and Pt). Although the strong influence of nutrients and organic matter on diatom community composition is well established (Jüttner et al., 2010; Stevenson et al., 2013), diatom-based metrics rarely take into account suspended particles for water quality assessment (but see Larras et al., 2017). Diatoms are indeed directly impaired by turbidity, reducing light availability for photosynthesis. Multimetric indices thus offer simple tools to summarize the effect of multi-pressure gradients on communities (Riato et al., 2018), and can be considered more effective for assessing biological conditions than a single metric (Stevenson et al., 2013). However, despite their increasing use, multimetric indices suffer from the subjectivity that can arise from metric selection (Reavie et al., 2008). Here, we attempted to avoid this pitfall by proposing a method of selecting metrics based on the robustness of their response to environmental gradients.
IBDL appears less stringent than IBML when assessing lakes’ ecological status. Literature comparing results from different indices in lakes, though scarce, tends to agree with this overestimation of water quality by diatom-based methods (Kolada et al., 2016). Phytobenthos has long been paid less attention than macrophytes for the assessment of lake ecological status. It is true that recent diatom-based metrics barely detected newly impacted lakes that would not have been detected by macrophyte metrics. Bennion et al. (2014) showed, for example, that their index (LTDI) performed well for lakes with good ecological status, but diatoms and other methods agreed less for lakes of lower status. This was particularly the case in the presence of morphological alterations, for which diatoms are poor indicators. A possible general explanation for the lower stringency of diatom-based indices in lakes is the high abundance of species complexes like Achnanthidium minutissimum or Gomphonema parvulum. Such complexes merge taxa that are morphologically close but with different ecological preferences. Due to the existence of different taxa within the A. minutissimum complex, many authors consider it an indicator of good water quality (Almeida et al., 2014), whereas others consider it tolerant toward toxic contaminants (micropollutants) and hydrologic disturbances (Cantonati et al., 2014; Lainé et al., 2014). Considering the generally high abundance of A. minutissimum in samples, this tends to blur the overall pressure-response relationship between index scores and environmental variables (Potapova & Hamilton, 2007). TITAN provides a means to avoid this pitfall, as such complexes are not selected as alert taxa, given that their abundance dynamics do not show clear response patterns to environmental gradients. Indeed, A. minutissimum, although highly abundant in our dataset (22% of total species abundances), was not considered an alert taxon.
The fact remains that IBDL tends to be less stringent than IBML, despite better relationships with Pt. In consequence, we have to explain why we think that the use of diatom-based indices to assess lake ecological status is justified.
First, the discrepancy between macrophyte and diatom responses relies mainly on the differences between their integration periods, given that indices provide information on ecological conditions over the time an assemblage develops. Lavoie et al. (2009) showed the integration period of diatom-based indices to be about 2–5 weeks for nutrients, whereas macrophytes react on yearly time scales (Kelly et al., 2016). As diatoms catch nutrients directly from the water column (Wetzel, 2001), they also may be more directly sensitive to rapid changes in trophic status than macrophytes (Vermaat et al., 2022). The rapid response of phytobenthos should justify its routine use (Schneider et al., 2019), in particular, for lakes in non-equilibrium states (Kelly et al., 2016).
Second, diatom-based indices are essential where hydrologic pressures in littoral areas prevent the development of macrophytes, and in lake typologies where macrophyte communities are naturally species poor or even absent (Schneider et al., 2019). Thus, while macrophyte-based indices cannot be calculated in all lakes, this is not true for diatom-based indices. Moreover, our results show that, with IBDL, water quality managers can directly compare ecological status assessments from different lakes even if the substrate sampled is different. Many studies highlighted that allelopathic relationships between macrophytes and epiphytic diatoms may be responsible for specific associations between macrophytes and diatom species and, thus, may contribute to the organization of particular assembly patterns (Hinojosa-Garro et al., 2010). In any case, in terms of ecological preferences, and consequently in terms of IBDL scores, our results did not show any significant differences between communities sampled on mineral substrates or macrophytes at the OU level, corroborating previous results obtained by Kitner and Poulíčková (2003) and Bennion et al. (2014). Other studies even support the use of epiphytic diatoms as biological indicators for lakes irrespective of the dominant macrophyte species sampled (Cejudo-Figueiras et al., 2010). The key point is to avoid senescent material or recently grown shoots that would potentially induce a colonization stage effect (King et al., 2006).
The next challenge was to check the consistency of the resulting classification of lakes based on IBDL to the harmonized definition of good ecological status established in the completed intercalibration exercise (Kelly et al., 2014b). The first step consisted in testing the correlation between IBDL scores and total phosphorus in our dataset. Only HA and MA typologies were considered here but, in any case, the last intercalibration exercise could not be performed for LA lakes. We obtained very good correlations that are clearly an improvement compared to the non-significant relationship previously obtained between BDI (diatom index used for the assessment of rivers) and Pt, and even better than the pressure-impact relationships observed at a pan-European scale (R2 between national methods and Pt ranged from 0.32 to 0.66 max., Kelly et al., 2014b). The second step consisted in testing the correlation between IBDL scores and the intercalibration common metric (CM) scores, in EQR. Here, the correlations demonstrated a very good agreement between IBDL and CM scores in both medium (R2 = 0.87) and high alkalinity (R2 = 0.82) lakes. We are, therefore, confident in our ability to match IBDL ecological status thresholds with those validated at the European level.
Conclusion
The new diatom index proposed here meets the requirements of the WFD and makes it possible to assess lakes’ ecological status in metropolitan France. The IBDL has proved to be particularly relevant as it has a twofold interest: an excellent relationship with total phosphorus and an application in any lake metatype. Its complementarity with IBML justifies the use of at least two primary producer components for ecological status classification (Kelly et al., 2016).
Availability of data and code
The data that support the findings of this study are openly available at https://doi.org/10.57745/PDKBGB.
References
AFNOR. (2016). Qualité de l’eau – Échantillonnage, traitement et analyse de diatomées benthiques en cours d'eau et canaux. Association française de normalisation, Norme NF 90 T-354.
AFNOR. (2022). Qualité de l’eau - Echantillonnage des communautés de macrophytes en plans d’eau. Norme NF T90–328.
Almeida, S. F., Elias, C., Ferreira, J., Tornés, E., Puccinelli, C., Delmas, F., & Sabater, S. (2014). Water quality assessment of rivers using diatom metrics across Mediterranean Europe: A methods intercalibration exercise. Science of the Total Environment, 476, 768–776.
Baker, M. E., & King, R. S. (2010). A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods in Ecology and Evolution, 1(1), 25–37.
Baker, E., King, R. S., & Kahle, D. (2020). TITAN2: Threshold indicator taxa analysis. R package version 2.4.1. Retrieved May, 15, 2023, from https://CRAN.R-project.org/package=TITAN2
Bennion, H., Kelly, M. G., Juggins, S., Yallop, M. L., Burgess, A., Jamieson, J., & Krokowski, J. (2014). Assessment of ecological status in UK lakes using benthic diatoms. Freshwater Science, 33(2), 639–654.
Birk, S., Bonne, W., Borja, A., Brucet, S., Courrat, A., Poikane, S., & Hering, D. (2012). Three hundred ways to assess Europe’s surface waters: An almost complete overview of biological methods to implement the Water Framework Directive. Ecological Indicators, 18, 31–41.
Boutry, S., Bertrin, V., & Dutartre, A. (2015). Indice Biologique Macrophytique en Lac (IBML): notice de calcul. Irstea, pp.25. ⟨hal-02602320⟩.
Brauer, V. S., Stomp, M., & Huisman, J. (2012). The nutrient-load hypothesis: Patterns of resource limitation and community structure driven by competition for nutrients and light. The American Naturalist, 179(6), 721–740.
Cantonati, M., & Lowe, R. L. (2014). Lake benthic algae: Toward an understanding of their ecology. Freshwater Science, 33(2), 475–486.
Carayon, D., Eulin-Garrigue, A., Vigouroux, R., & Delmas, F. (2020). A new multimetric index for the evaluation of water ecological quality of French Guiana streams based on benthic diatoms. Ecological Indicators, 113, 106248.
Cejudo-Figueiras, C., Alvarez-Blanco, I., Bécares, E., & Blanco, S. (2010). Epiphytic diatoms and water quality in shallow lakes: The neutral substrate hypothesis revisited. Marine and Freshwater Research, 61(12), 1457–1467.
Cellamare, M., Morin, S., Coste, M., & Haury, J. (2012). Ecological assessment of French Atlantic lakes based on phytoplankton, phytobenthos and macrophytes. Environmental Monitoring and Assessment, 184(8), 4685–4708.
CEN (Comité Européen de Normalisation). (2003). Water quality - Guidance standard for the routine sampling and pretreatment of benthic diatoms from rivers. EN 13946:2003. Comité Européen de Normalisation, Geneva, Switzerland.
Costas, N., Pardo, I., Méndez-Fernández, L., Martínez-Madrid, M., & Rodríguez, P. (2018). Sensitivity of macroinvertebrate indicator taxa to metal gradients in mining areas in Northern Spain. Ecological Indicators, 93, 207–218.
Coste, M., Boutry, S., Tison-Rosebery, J., & Delmas, F. (2009). Improvements of the biological diatom index (BDI): Description and efficiency of the new version (BDI-2006). Ecological Indicators, 9(4), 621–650.
Dufrêne, M., & Legendre, P. (1997). Species assemblages and indicator species: The need for a flexible asymmetrical approach. Ecological Monographs, 67(3), 345–366.
European Union. (2000). Directive 2000/60/EC of the european parliament and of the council of 23rd october 2000 establishing a framework for community action in the field of water policy. Official journal of european communities, european commission, brussels (2000) (22 December, L 327/1).
Gieswein, A., Hering, D., & Lorenz, A. W. (2019). Development and validation of a macroinvertebrate-based biomonitoring tool to assess fine sediment impact in small mountain streams. Science of the Total Environment, 652, 1290–1301.
Gonzalez-Paz, L., Delgado, C., & Pardo, I. (2020). Understanding divergences between ecological status classification systems based on diatoms. Science of the Total Environment, 734, 139418.
Gotelli, N. J., & McCabe, D. J. (2002). Species co-occurrence: A meta-analysis of J.M. Diamond's assembly rules model. Ecology, 83(8), 2091–2096.
Hinojosa-Garro, D., Mason, C. F., & Underwood, G. J. (2010). Influence of macrophyte spatial architecture on periphyton and macroinvertebrate community structure in shallow water bodies under contrasting land management. Fundamental and Applied Limnology, 177(1), 19–28.
Kelly, M., Urbanic, G., Acs, E., Bennion, H., Bertrin, V., Burgess, A., Denys, L., Gottschalk, S., Kahlert, M., Karjalainen, S., Kennedy, B., Kosi, G., Marchetto, A., Morin, S., Picinska-Fałtynowicz, J., Poikane, S., Rosebery, J., Schoenfelder, I., Schoenfelder, J., & Varbiro, G. (2014a). Comparing aspirations: Intercalibration of ecological status concepts across European lakes for littoral diatoms. Hydrobiologia, 734, 125–141.
Kelly, M., Acs, E., Bertrin, V., Bennion, H., Borics, G., Burgess, A., Denys, L., Ecke, F., Kahlert, M., Karjalainen, S., Kennedy, B., Marchetto, A., Morin, S., Picinska Faltynowicz, J., Phillips, G., Schönfelder, I., Schönfelder, J., Urbanic, G., Van Dam, H., & Zalewski, T. (2014b). Water Framework Directive Intercalibration Technical Report: Lake Phytobenthos ecological assessment methods Publications Office of the European Union, 125.
Kelly, M. G., Birk, S., Willby, N. J., Denys, L., Drakare, S., Kahlert, M., Karjalainen, S. M., Marchetto, A., Pitt, J.-A., Urbanic, G., & Poikane, S. (2016). Redundancy in the ecological assessment of lakes: Are phytoplankton, macrophytes and phytobenthos all necessary? Science of the Total Environment, 568, 594–602.
Khamis, K., Hannah, D. M., Brown, L. E., Tiberti, R., & Milner, A. M. (2014). The use of invertebrates as indicators of environmental change in alpine rivers and lakes. Science of the Total Environment, 493, 1242–1254.
King, L., Clarke, G., Bennion, H., Kelly, M., & Yallop, M. (2006). Recommendations for sampling littoral diatoms in lakes for ecological status assessments. Journal of Applied Phycology, 18, 15–25.
King, R. S., & Richardson, C. J. (2003). Integrating bioassessment and ecological risk assessment: An approach to developing numerical water-quality criteria. Environmental Management, 31(6), 795–809.
Kitner, M., & Poulícková, A. (2003). Littoral diatoms as indicators for the eutrophication of shallow lakes. Hydrobiologia, 506(1), 519–524.
Kolada, A., Pasztaleniec, A., Bielczyńska, A., & Soszka, H. (2016). Phytoplankton, macrophytes and benthic diatoms in lake classification: Consistent, congruent, redundant? Lessons learnt from WFD-compliant monitoring in Poland. Limnologica, 59, 44–52.
Krammer, K., & Lange-Bertalot, H. (1986–1991). Bacillariophyceae. Suswasserflora von Mitteleuropa. Gustav Fisher Verlag.
Jüttner, I., Chimonides, P. J., & Ormerod, S. J. (2010). Using diatoms as quality indicators for a newly-formed urban lake and its catchment. Environmental Monitoring and Assessment, 162, 47–65.
Lainé, M., Morin, S., & Tison-Rosebery, J. (2014). A multicompartment approach -diatoms, macrophytes, benthic macroinvertebrates and fish- to assess the impact of toxic industrial releases on a small French river. PLoS ONE, 9(7), e102358.
Lange-Bertalot, H. (1995–2015). Iconographia Diatomologica. Annotated Diatom Micrographs. Koeltz Scientific Books.
Lange-Bertalot, H. (2000-2013). Diatoms of Europe - Diatoms of the European inland waters and comparable habitats. Konigstein: Koeltz Scientific Books.
Lavoie, I., Hamilton, P. B., Wang, Y. K., Dillon, P. J., & Campeau, S. (2009). A comparison of stream bioassessment in Québec (Canada) using six European and North American diatom-based indices. Nova Hedwigia, 135, 37–56.
Larras, F., Coulaud, R., Gautreau, E., Billoir, E., Rosebery, J., & Usseglio-Polatera, P. (2017). Assessing anthropogenic pressures on streams: A random forest approach based on benthic diatom communities. Science of the Total Environment, 586, 1101–1112.
Lecointe, C., Coste, M., & Prygiel, J. (1993). “Omnidia”: Software for taxonomy, calculation of diatom indices and inventories management. Hydrobiologia, 269, 509–513.
Mondy, C. P., Villeneuve, B., Archaimbault, V., & Usseglio-Polatera, P. (2012). A new macroinvertebrate-based multimetric index (I2M2) to evaluate ecological quality of French wadeable streams fulfilling the WFD demands: A taxonomical and trait approach. Ecological Indicators, 18, 452–467.
Morin, S., Valade, D., Tison-Rosebery, J., Bertrin, V., Cellamare, M., & Dutartre, A. (2010). Utilisation du phytobenthos pour la bioindication en plans d’eau : Etat de l’art des méthodes disponibles et test de métriques sur les plans d'eau aquitains. Rapport scientifique, Irstea.
Poikane, S., Kelly, M., & Cantonati, M. (2016). Benthic algal assessment of ecological status in European lakes and rivers: Challenges and opportunities. Science of the Total Environment, 568, 603–613.
Potapova, M., & Hamilton, P. B. (2007). Morphological and ecological variation within the Achnanthidium minutissimum (Bacillariophyceae) species complex. Journal of Phycology, 45, 561–575.
R Core Team. (2021). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Retrieved May, 15, 2023, from http://www.R-project.org/
Reavie, E. D., Kireta, A. R., Kingston, J. C., Sgro, G. V., Danz, N. P., Axler, R. P., & Hollenhorst, T. P. (2008). Comparison of simple and multimetric diatom-based indices for great lakes coastline disturbance. Journal of Phycology, 44(3), 787–802.
Riato, L., Leira, M., Della Bella, V., & Oberholster, P. J. (2018). Development of a diatom-based multimetric index for acid mine drainage impacted depressional wetlands. Science of the Total Environment, 612, 214–222.
Rott, E., Pipp, E., Pfister, P., van Dam, H., Ortler, K., Binder, N., & Pall, K. (1998). Indikationslisten fur Aufwuchsalgen. In M. Scheffer (Ed.), Ecology of shallow lakes. Chapman and Hall.
Schneider, S. C., Hjermann, D. O., & Edvardsen, H. (2019). Do benthic algae provide important information over and above that provided by macrophytes and phytoplankton in lake status assessment?–Results from a case study in Norway. Limnologica, 76, 28–40.
Stenger-Kovács, C., Buczko, K., Hajnal, E., & Padisák, J. (2007). Epiphytic, littoral diatoms as bioindicators of shallow lake trophic status: Trophic Diatom Index for Lakes (TDIL) developed in Hungary. Hydrobiologia, 589(1), 141–154.
Stevenson, J. (2014). Ecological assessments with algae: A review and synthesis. Journal of Phycology, 50, 437–461.
Stevenson, R. J., Zalack, J. T., & Wolin, J. (2013). A multimetric index of lake diatom condition based on surface-sediment assemblages. Freshwater Science, 32(3), 1005–1025.
Venables, W. N., & Ripley, B. D. (2002). Modern applied statistics with S (4th ed.). Springer.
Vermaat, J. E., Biberdžić, V., Braho, V., Gjoreska, B. B., Cara, M., Dana, Z., & Schneider, S. C. (2022). Relating environmental pressures to littoral biological water quality indicators in Western Balkan lakes: Can we fill the largest gaps? Science of the Total Environment, 804, 150–160.
Wetzel, R. G. (2001). Protists: Key ecosystem regulators. BioScience, 51(12), 997.
Wickham, H. (2022). Tools for working with URLs and HTTP. R package version 1.4.4. Retrieved May, 15, 2023, from https://CRAN.R-project.org/package=httr
Zelinka, M., & Marvan, P. (1961). Zur Priizisierung derbiologischen Klassification der Reinheit fliessender Gewàsser. Archiv Für Hydrobiology, 57, 389–407.
Acknowledgements
We thank all Water Agencies for data sharing and all Regional Departments for Environment for data collection. We also thank the two reviewers for their helpful comments on this work.
Funding
The research leading to these results received funding from the French Biodiversity Agency (OFB, pôle ECLA).
Author information
Authors and Affiliations
Contributions
All authors participated in designing the study and developing aims and research questions. S.B. designed methodology, extracted data and made the analyses, supported by T.L. concerning pretreatments before intercalibration. J.T.R. led the writing of the manuscript supported by S.B., S.M., and V.B. All authors contributed critically to the drafts, contributed to the final version of the manuscript, and gave final approval for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tison-Rosebery, J., Boutry, S., Bertrin, V. et al. A new diatom-based multimetric index to assess lake ecological status. Environ Monit Assess 195, 1202 (2023). https://doi.org/10.1007/s10661-023-11855-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11855-w