Abstract
The current study investigated the use of VNIR–SWIR (visible/near infrared to short-wavelength infrared: 400–2500 nm) spectroscopy for predicting trace metals in overbank sediments collected in the study site. Here, we (i) derived spectral absorption feature parameters (SAFPs) from measured ground spectra for correlation with trace metal (Pb, Cd, As, and Cu) contents in overbank sediments, (ii) built univariate regression models to predict trace metal concentrations using the SAFPs, and (iii) evaluated the predictive capacities of the regression models. The derived SAFPs associated with goethite in overbank sediments were Depth433b, Asym433b, and Width433b, and those associated with kaolinite in overbank sediments were Depth1366b, Asym1366b, Width1366b, Depth2208b, Asym2208b, and Width2208b. Cadmium in the overbank sediments showed the strongest correlations with the goethite-related SAFPs, whereas Pb, As, and Cu showed strong correlations with goethite- and kaolinite-related SAFPs. The best predictive models were obtained for Cu (R2 = 0.73, SEE = 0.15) and Pb (R2 = 0.73, SEE = 0.21), while weaker models were obtained for As (R2 = 0.46, SEE = 0.31) and Cd (R2 = 0.17, SEE = 0.81). The results suggest that trace metals can be predicted indirectly using the SAFPs associated with goethite and kaolinite. This is an important benefit of VNIR–SWIR spectroscopy considering the difficulty in analyzing “trace” metal concentrations, on large scales, using conventional geochemical methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Trace metal pollution in the environment is one of the major concerns related to acid mine drainage (AMD). This is because trace metals do not chemically degrade and thus, can accumulate in hazardous concentrations in the environment. The accumulation of trace metals over time poses potential threat, not only to the environment and animal species, but also to human health via contaminated soil and water resources (N’Guessan et al., 2009; Wu et al., 2005; Xie et al., 2012). Thus, the detection and monitoring of trace metals in AMD-contaminated areas is crucial for mitigation of the hazard they pose to human, societal, and environmental health (Bradshaw, 2000).
Traditional wet chemical methods for detecting and monitoring trace metal contamination in the environment involve a number of, often tedious and time-consuming, steps such as (Pandit et al., 2010; Wu et al., 2007): (i) sample collection, preservation, and preparation for analysis, (ii) destructive and costly laboratory analysis, and (iii) the continuous reproduction of geochemical maps. Thus, in cases where rapid data collection and analyses are necessary to detect and monitor trace metal contamination associated with mining accidents (Kemper & Sommer, 2002, 2003) and natural disasters in mining areas (McCarthy & Humphries, 2012), the conventional geochemical methods become inefficient and costly. Consequently, there is a need for a more efficient and cost-effective method for timeous detection and monitoring of trace metal contamination in the environment.
Reflectance spectroscopy is a promising tool that offers a non-destructive, in-situ, easily reproducible, and potentially cost-effective method for predicting trace metal concentrations in the environment (Wu et al., 2007). It is the study of surface materials’ interaction with (i.e., scattering and absorption of) light (Mustard & Glotch, 2020). The proportion of light that is scattered and absorbed is largely controlled by the chemical composition and structure of the material interacted with, thus, generating distinct spectral absorption features (SAFs) for that material (Bishop, 2020). SAFs in the visible/near infrared (VNIR) to short-wavelength infrared (SWIR) region (400–2500 nm) of the electromagnetic (EM) spectrum are known to be diagnostic of soil properties and mineralogy (Ben-Dor et al., 1999; Lilliesand et al., 2015). SAFs in this region of the EM spectrum are mainly the result of electronic transitions and overtones and combinations of fundamental molecular vibrations of the crystal lattice (Bishop, 2020).
Trace metals at concentrations below 4000 ppm are spectrally inactive in the VNIR–SWIR region of the EM spectrum (Wu et al., 2007). However, they do exhibit distinct SAFs when bound to spectrally active soil components such as clays (Clark, 1999; Van der Meer, 1999) and iron oxides (Ben-Dor et al., 1999). Clay-related SAFs are associated with molecular vibrations between 1300 and 2500 nm (Clark, 1999) while iron oxide-related SAFs are mainly the result of crystal field effects and charge transfer between transition metals and related ligands between 400 and 1200 nm of the EM spectrum (Ben-Dor et al., 1999). As trace metal cations are adsorbed to surface hydroxyl (OH) groups on clays and metal oxides, H+ is released, thus, decreasing the number of OH sites and increasing the number of oxygen (O) sites on the mineral surface (Schindler & Sposito, 1991). The decrease in OH and increase in O sites on oxide and clay surfaces may cause changes in the area, depth, and asymmetry of their absorption peaks (here, referred to as SAFPs), thus allowing the indirect quantification of trace metals (Choe et al., 2008).
Numerous authors have demonstrated the potential of VNIR–SWIR spectroscopy for predicting trace/heavy metal contents in agricultural and mine soils (Gholizadeh et al., 2015; Ji et al., 2010; Omran, 2016; Pandit et al., 2010; Ren et al., 2009; Sawut et al., 2018; Song et al., 2015; Tu et al., 2021; Wang et al., 2022; Xie et al., 2012; Zhang et al., 2019; Zhang et al., 2022), lake- (Jiang et al., 2018; Malley & Williams, 1997), and stream sediments (Choe et al., 2008; Piroozfar et al., 2018). However, trace/heavy metal prediction in overbank/floodplain soils and sediments has received only limited attention (Lamine et al., 2019). In addition, no research has yet investigated the potential of field VNIR–SWIR spectroscopy for predicting trace/heavy metals in sediments along streams in coal mining districts. Coal mine wastes are different to that of metal mines chiefly because coal is a sedimentary deposit which, compared to non-sedimentary metallic deposits and the common metal sulfides therein, are largely dominated by pyrite (Eby, 2004; INAP, 2009). As a result, coal mine drainage is typified by elevated iron (Fe), aluminum (Al), and manganese (Mn) and trace metal concentrations related to their sedimentary strata (INAP, 2009).
Here, we assess the feasibility of using ground (field) VNIR–SWIR spectroscopy to predict trace metal contents in overbank sediments along an AMD-contaminated stream draining a coal mine. The objectives of this study were to (i) derive SAFPs from ground spectra and relate these to trace metal contents in overbank sediments in the study site, (ii) construct univariate regression models to predict trace metal concentrations using the derived SAFPs, and (iii) evaluate the stability and predictive capacities of the regression models.
Materials and methods
Description of the study site
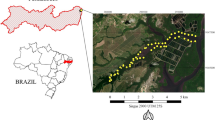
The Blesbokspruit River is a low order stream that forms part of the Olifants River catchment in South Africa. It is located roughly 5 km NW of the town of Emalahleni in Mpumalanga province. Emalahleni is well known for its long history of coal mining (Bell et al., 2001). The Blesbokspruit River was chosen as an area of interest because (i) prior AMD-related studies have been conducted at this site (Bell et al., 2001; Netshitungulwana et al., 2013) and (ii) the site is not completely overgrown with vegetation and thus, it comprises several areas with exposed overbank sediments that are suitable for the measurement of ground spectra. The study site has four constructed acid pools located at the headwaters of the stream and a wetland located roughly 3 kms downstream of the acid pools (Fig. 1).
Localities (red dots) for overbank sediment sampling and ground hyperspectral data collection along the Blesbokspruit River, Mpumalanga, South Africa. Overbank sediment samples were collected at two sites roughly 5 m apart at each of the six different localities. Flow direction is indicated by the black, dashed arrow. Also shown is a wetland (green dash lines) and acid ponds (yellow rectangle)
Sample collection
Twelve overbank sediment samples (i.e., two samples roughly 5 m apart at six different localities) were collected along an approximately 6 km section of the Blesbokspruit River (Fig. 1). The collection of two overbank sediment samples roughly 5 m apart at each of the six different localities was in view of the knowledge that overbank sediment deposition rates show considerable spatial variability (Walling & He, 1998). The roughly 5 m separation from sample pairs at of the six different location was considered adequate considering that knowledge gained from this study will be imported to the subsequent analysis of 1-m spatial resolution airborne reflectance hyperspectral data that are available over the study area.
Samples were collected during autumn, one of the driest seasons of the year in the study area, during which moisture levels in overbank sediments are low and thus, ideal for the measurement of spectra in the field (Wu et al., 2005). The sampled section of the river was limited to the first 6 km downstream from the acid pools because of the neutralizing and diluting effect of the Prison tributary roughly 5 km downstream of the acid pools (Bell et al., 2001). Sample collection along the stream was also restricted by the presence of a wetland (Fig. 1) and sewage contamination from a neighboring informal settlement.
In geology and environmental sciences, more often than not, sample design and density are beyond the control of the researcher (Davis, 2002). Thus, geological and environmental data are occasionally sparse (e.g., n = 4 in Dragović et al., 2010; n = 6 in Mutiyar & Mittal, 2014; n = 3 in Baran & Tarnawski, 2015). In these situations, data must be collected where available and appropriate statistical analyses must be applied (Davis, 2002). In our case, the sparseness of data (due to financial, time, and field constraints) was addressed by applying statistical analyses that are appropriate for a small set of data and the uncertainty determined using confidence intervals (CIs) and statistical significance (p). While larger datasets are preferred over smaller ones (de Winter, 2013), they are not without their challenges. They can introduce arbitrary “spurious” correlations far exceeding the meaningful ones (Poppelars, 2015). As a result, the number of significant correlations are often exaggerated for larger datasets (Calude & Longo, 2017), thereby potentially impeding subsequent predictions. Here, we considered our samples n = 12 to be adequate for simple univariate regression (see the “Data analysis” section below) because, according to Van Voorhis and Morgan (2007), the absolute minimum sample size is n = 10 per predictor.
Geochemical analysis
The sediment samples were air-dried at the base camp to limit possible changes in redox, and were passed through a 63 μm nylon sieve to separate the clay-and silt sized fraction. This size fraction has been considered the most important host of trace metals in soils and sediments (Förstner & Salomons, 1980). Nylon sieves and high density polyethylene storage bags were used to avoid possible contamination with metals targeted in this study (Zief & Mitchell, 1976). In the laboratory, the sieved samples were pretreated with reverse aqua regia (which excludes trace metals bound in the crystal lattice of primary minerals and releases the environmentally extractable proportions of trace metals) (Shahbazi & Beheshti, 2019) and then decomposed using microwave digestion for analysis by ICP-AES/MS. The samples were analyzed for major elements (Al, Fe, Si, and Mn) and trace metal (loid)s (Cu, Pb, Cd, and As) because they are often closely related to AMD (España, 2007; Nieto et al., 2007; Sengupta, 1993). The following were used in this study for quality assurance and quality control purposes: (i) field and analytical duplicates, (ii) procedural blanks, and (iii) soil certified reference materials (CRM). Analytical results for elements with precision of 20% or better (Ramsey, 1998) were retained for further data analysis.
Mineralogical analysis
For XRD Rietveld analysis, overbank sediment samples were crushed and split, and subsamples were milled to obtain a size fraction less than 75 μm. Milling was necessary to ensure that (Buhrke et al., 1998): (i) crystallite orientations were randomized, (ii) there were adequate quantities of crystallites to yield a representative intensity distribution for a given sample, and (iii) adequate diffraction intensity was yielded to meet counting statistics. The milled samples were then analyzed using the Bruker D8 Advance diffractometer. The samples were not separated into various soil fractions prior to the XRD analysis to ensure that the results of this study will be consistent with the subsequent analysis of airborne hyperspectral data collected over the study area that is not discussed here but in a future publication.
Ground spectral analysis
Using a portable ASD FieldSpec® 3 spectroradiometer, four ground spectral measurements were collected (because of the relative stability of measurements) at each of the twelve sample sites in six different localities (Fig. 1). The instrument used measures reflectance spectra across the VNIR–SWIR (350–2500 nm) region of the EM spectrum. Spectral data were measured in-situ (i.e., not dried prior to measurement) to ensure consistency with airborne hyperspectral data that are available over the study area. Spectra were measured under clear skies, with data collection restricted to 11 AM and 2 PM each day, when the sun was at or closest to its peak (Goetz, 2012). A field of view of 25° was used because this is considered best for ground spectral measurements (Janse et al., 2018) and a white reference panel (Spectralon) was used in between measurements as a baseline for the spectral measurements. The Spectralon was made of polytetrafluoroethylene and cintered halon (ASD Inc., 2009). This material is known to be nearly 100% reflective within the VNIR–SWIR wavelength range, scattering light uniformly in all directions within that wavelength range (ASD Inc., 2009).The ground spectra were captured using the RS3 software package included with the ASD FieldSpec® 3 spectroradiometer.
Preprocessing
Because of the small number of measurements (n = 4) collected at each sample location, a combined spectral plot (Fig. 2) was generated using the median as an estimator of central tendency. The advantage of using the median over the mean is that (i) it is robust against outliers and (ii) it does not make any distributional assumptions, making it better suited for skewed data as is common in exploration and environmental geochemistry (Reimann & Filzmoser, 2000). There is a noticeable offset in spectra around 1000 nm (Fig. 2). This offset is a common problem associated with hyperspectral data when the same wavelength is measured by more than one sensor (Grillini et al., 2021). In our case, the offset is the result of the spectral overlap between the VNIR detector (measuring 350–1000 nm) and SWIR1 detector (measuring 1000–1800 nm) in the ASD FieldSpec® 3 spectroradiometer (ASD Inc., 2009).
Median raw ground-derived spectra of overbank sediments at each of the 12 sample sites (Fig. 1) along the Blesbokspruit River, highlighting the wavelengths associated with atmospheric water
Spectra between 1830 and 1930 nm, and between 2350 and 2500 nm (Fig. 2) were removed to exclude the noise associated with atmospheric water (Clevers et al., 2008; Pandit et al., 2010; Prasad et al., 2015). The remaining reflectance data were then enhanced using continuum-removal (CR), which is a widely used transformation in spectroscopy (Piroozfar et al., 2018; Prasad & Gnanappazham, 2016; Zhao et al., 2020). In CR analysis, the overall albedo of a reflectance curve (called the continuum) is removed, thereby scaling reflectance spectra to 100% when approaching the continuum (Van der Meer, 2004). Spectral absorption features do not occur at every wavelength within the VNIR–SWIR range but rather at certain wavelengths typically associated with oxides, clays, carbonates, sulfides, and organic matter (Malley & Williams, 1997). Here, changes in SAFPs, including absorption-band position, absorption-band depth (D), absorption-band width (W), and absorption-band asymmetry (S), were derived from the strongest SAFs in the CR spectra (Fig. 3) around 433 nm, which are known to be associated with lattice OH in goethite (Balsam & Wolhart, 1993), and around 1400 and 2200 nm, which are known to be associated with OH and Al–OH groups, respectively, related to kaolinite (Hunt & Ashley, 1979; Khunsa et al., 2017; Van der Meer, 1999).
Continuum-removed ground spectra of overbank sediments at each of the 12 sample sites (Fig. 1) along the Blesbokspruit River, with wavelengths related to atmospheric water removed and the spectral subsets used to derive the SAFPs enclosed in black rectangles
Definition of the SAFPs
Absorption-band position, D, S, and W can be calculated from CR spectra according to Fig. 4. Absorption-band position is the wavelength corresponding with the minimum reflectance percentage over the wavelength range of the absorption feature (Van der Meer, 2004).
Definition of absorption-band position, depth, and asymmetry (modified after Van der Meer, 1999)
The D is typically defined relative to the hull/continuum (Rc), as:
where Rb is the reflectance at the wavelength minimum and Rc is the reflectance of the continuum at the wavelength position of Rb (Van der Meer, 2004). The S is typically defined as:
where the Arealeft is the area from the starting point of the absorption feature to the minimum reflectance point and Arearight is the area from the minimum reflectance point to the shoulder (end point) of the absorption feature (Van der Meer, 2004). The W is typically defined as the area to the left and right of the minimum reflectance value/percentage of the absorption feature relative to the absorption depth (Van der Meer, 1999), thus:
Data analysis
Of the spectral data measured, all but the spectra in the subset around 2200 nm for samples no. 1 and 2 were used in further statistical analysis. Spectra measured around 2200 nm for samples no. 1 and 2 were excluded from the statistical analysis because they were very noisy and likely a result of the high percentage of local humidity (i.e., 24%) at the time and day of measurement. Because of the small number of overbank sediment samples (n = 12), correlations among the CR SAFPs were determined using Spearman’s rank correlation analysis, which is suitable for smaller datasets and is robust against outliers (Reimann & Filzmoser, 2000). The reliability of the correlations is expressed by the statistical significance (p) and confidence intervals (CIs). Although the trace metal concentrations are compositional data (Buccianti & Pawlowsky-Glahn, 2005; Pawlowsky-Glahn & Egozcue, 2006), they were not subjected to log-ratio transformation because the data per element were correlated with spectral data which do not come from the same, closed composition, thus, rendering them independent (Reimann & de Caritat, 2017). However, the trace metal concentration data were ln-transformed to improve the normality of the data prior to further analysis (Reimann & Filzmoser, 2000). The relationships between trace metal concentration data (as target variables) and spectral data (as predictor variables) can be modeled using linear regression analysis (i.e., one predictor variable (or regressor) and one target variable (or regressand). However, multiple linear regression analysis is unsuitable when predictor variables show high collinearity, as is typical of spectral data (Van der Meer & Jia, 2012). In addition, the resulting models are often too complex to understand (Wu et al., 2005).
Here, SAFPs which showed the strongest correlations with trace metal contents were used to establish univariate regression models, thereby, satisfying the “one in ten” rule of thumb for the number of samples required per predictor variable in regression analysis (Austin & Steyerberg, 2015; Harrell Jr., 2001; Steyerberg, 2009). Calibration regression models were evaluated using the coefficient of determination (R2) and the standard error of estimation (SEE) as fitness indicators. The goodness of fit increases as R2 values approach 1 while the uncertainty of the calibration models decrease as SEE values approach 0. “Predicted” concentrations were calculated using the regression equation obtained for each of the calibration regression models. Predicted concentrations in ln were then back-transformed to normal values for comparison with measured trace metal concentrations. The predictive capacity of each of the regression models was evaluated using the leave-one-out cross-validation (LOO-CV) method, which is suitable for very small datasets (Yadav & Shukla, 2016). Here, a single data point was removed from the dataset and the regression analysis performed using the remaining data points. The resulting model was then used to predict the removed data point and the squared error (SE) was calculated for the predictive models. This process was repeated until each of the data points has been removed and used for cross-validation. The prediction capacity was determined by calculating the root mean squared error of prediction of cross-validation (RMSEPCV). The stability of the predictive models was evaluated using the Chow test (Chow, 1960), which examines the equality of regression coefficients (i.e., slope and intercept) across subsets of the data and returns a significant result if the coefficients are statistically different (Sotirakopoulos et al., 2015).
Results and discussion
Mineralogy and geochemistry
Table 1 shows the mineralogical and trace metal composition of the overbank sediment samples collected from the study site. The results of XRD Rietveld analysis showed that the overbank sediments in the study site contained mainly quartz (95–100%) and kaolinite (0–5%). Thus, they can be considered as sediments with sandy texture. The major element composition of the overbank sediment samples can be summarized according to decreasing median concentrations (ppm) as: Al (44719.56) > Fe (25670.24) > Si (3459.86) > Mn (212.17). The trace metal composition of the overbank sediment samples can be summarized according to decreasing median concentrations (ppm) as: Cu (31.77) > Pb (25.21) > As (7.40) > Cd (0.03). The major element composition of the overbank sediments suggests that the < 63 μm fraction of the overbank sediments is largely dominated by Al and Fe oxides and oxyhydroxides, with lower proportions of clay (indicated by Si) and minor proportions of Mn oxides and oxyhydroxides.
Correlations among the derived goethite- and kaolinite-related SAFPs and ln-transformed trace metal contents are shown in Table 2. The rationale for trace metal predictions is based on the knowledge that iron oxide minerals (Parker et al., 2007; Webster et al., 1998), such as goethite, and clay minerals (Uddin, 2017; Ugwu & Igbokwe, 2019), such as kaolinite, play significant roles in trace metal attenuation in aquatic environments, largely via adsorption processes. According to Table 2, Cd showed strong (r > 0.7) and significant (CI > 95%) correlation with only the goethite-related SAFP (Depth433). This strong correlation is supported by Covelo et al. (2007) who determined that Cd was preferentially adsorbed (and retained) by Fe-oxides, compared to clays, in soils. Pb showed strong (r > 0.7) and significant (CI > 95%) correlations with both the goethite-related SAFPs (Depth433 and Width433) and the kaolinite-related SAFP (Width2208). These strong correlations are consistent with Moreno et al. (2006) who found that iron oxide and clay (the most significant of which was kaolinite) contents played important roles in the adsorption of Pb. Arsenic appeared strongly correlated (r > 0.7) with both goethite-related (Asym433) and kaolinite-related (Asym2208) SAFPs. While the strong and significant correlation with goethite is expected (Kumpiene et al., 2008; Palansooriya et al., 2020), the strong correlation with kaolinite (Asym2208) is consistent with Choe et al. (2009) and Piroozfar et al. (2018) and may be attributed to strong complexes between As and (i) octahedrally coordinated aluminum- (Halter & Pfeifer, 2001) and (ii) Fe and Al hydroxide coatings on kaolinite surfaces (Goldberg, 2002). Similar to As, Cu is strongly correlated (r > 0.7) with both goethite- (Asym433) and kaolinite-related (Depth1366, and Width1366) SAFPs. Copper is commonly associated with Fe oxides (Kabata-Pendias & Pendias, 2000; Kumpiene et al., 2008) and similar to As, has shown a strong affinity for kaolinite when it is coated with Fe hydroxides (González-Costa et al., 2017; Osei & Singh, 2000; Zhuang & Yu, 2002), as is applicable in the study area.
Regression modeling
Model calibration
Inputs to the univariate regression modeling were based on correlations of the SAFPs with the ln-transformed trace metal concentrations (Table 2). Table 3 shows the R2 and SEE of the calibration models obtained for Pb, Cd, As, and Cu. The SEE generally provides a better estimate of a model’s predictive accuracy, compared to the R2, because it is a measure of the actual distance of the data points from the regression line on average (Frost, 2023a). Thus, according to the SEE, the best performing model was obtained for Cu (SEE = 0.15), followed by Pb (SEE = 0.21), As (SEE = 0.31), and Cd (SEE = 0.81).
Model evaluation
Table 4 shows the regression equations, SE, RMSEPCV and Chow statistic (p) for Pb, Cd, As, and Cu, using the LOO-CV method. According to the Chow statistic (Table 4), regression coefficients generated by CV, for each of the trace metals, were stable (p > 0.05) and thus, robust, in spite of the small sample size. According to Table 4, the lowest RMSEP was obtained for Cd (0.06), followed by As (1.70), Cu (2.50), and Pb (4.30). However, when the RMSEP is compared with the concentration range of each of the metals, the RMSEP obtained for Cd represents ~ 25% of the population; for As, it represents ~ 17% of the population; for Cu, it represents ~ 9% of the population and for Pb, it represents ~ 14% of the population. Thus, Cu was the most accurately predicted while Cd was the least accurately predicted.
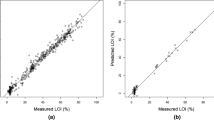
Scatterplots depicting measured vs. predicted trace metal (Pb, Cd, As, and Cu) contents were generated as part of the predictive model evaluation process (Fig. 5). Data points appeared mostly scattered in pairs (generally corresponding with the 5-m-spaced sample pairs). According to Fig. 5, the highest R2 was obtained for Cu (R2 = 0.73) and Pb (R2 = 0.73), followed by Cd (R2 = 0.70) and As (R2 = 0.46). The R2 values obtained for Pb (Fig. 5a) and Cu (Fig. 5d) are in good agreement with the distribution of points around their 1:1 control lines. However, the regression line obtained for Cd (Fig. 5b) showed significant deviation from the 1:1 control line, suggesting that its high R2 is likely strongly influenced by the outliers at ~ 0.25 ppm. When the outliers are removed from the regression analysis, the R2 lowers substantially from 0.7 to 0.17. This suggests that the Cd model is inappropriate for predictions and thus, is a limitation of this study.
The RMSEP (Table 4), which generally shows a high sensitivity to outliers (Frost, 2023b), is a seemingly unsuitable estimation of model performance, especially for Cd. In addition, it was strongly controlled by the concentration range of the training dataset (i.e., Cd had the lowest RMSEP because its training dataset had the lowest mean) and does not agree with the regression line obtained for Cd (Fig. 5b). In contrast, the SEE (Table 3), showed better consistency with the regression line obtained for Cd, compared to the RMSEP, and thus, is a more suitable estimate of model accuracy.
Conclusions
Conventional geochemical methods of investigating trace metal contents in soils and sediments can be very costly and time-consuming, especially when conducted on large scales. This study served as a first attempt to predict potentially toxic trace metals in overbank sediments of the Witbank Coalfield using VNIR–SWIR spectroscopy and the distinct SAFPs of mineral-bound trace metals. We determined the following:
-
1.
The strongest calibration models were obtained using Depth433, Depth1366, Width2208, and Asym2208 as trace metal predictors. The association of these predictors with goethite and kaolinite suggests that these minerals play an important role in trace metal attenuation and prediction in the study area.
-
2.
Of the trace metals analyzed, Cu and Pb were the easiest to predict while As and Cd were harder to predict.
-
3.
The RMSEP was highly sensitive to outliers, as well as the concentration ranges of the analyzed metals. Thus, it provided a seemingly inappropriate estimate of predictive model accuracy. In contrast, the SEE appeared more consistent with the regression lines obtained for the predictive models and thus, was a better estimate of the predictive accuracy, overall.
Although the data analysis was tailored to the small sample size, it remains a limitation of this work. This study, therefore, serves mainly as a proof of concept. Additional research using a larger dataset and more complex multivariate regression analysis is warranted to improve the validation of the predictive models. Following improved validation, ground reflectance spectroscopy could prove to be a valuable screening tool for detecting trace metal concentrations in overbank sediments with significant Fe oxides and clays, as a precursor to more in-depth sampling and geochemical analyses.
Data availability
The data that support the findings of this study are available from the corresponding author, Jamie-Leigh Robin Abrahams, upon reasonable request.
References
ASD Inc. (2009). FieldSpec®3 user manual. ASD Document 600540 Rev. H.
Austin, P. C., & Steyerberg, E. W. (2015). The number of subjects per variable required in linear regression analyses. Journal of Clinical Epidemiology, 68(6), 627–636. https://doi.org/10.1016/j.jclinepi.2014.12.014
Balsam, W. L., & Wolhart, R. (1993). Sediment dispersal in the Argentine Basin: Evidence from visible light spectra. Deep Sea Research Part II: Topical Studies in Oceanography, 40, 1001–1031.
Baran, A., & Tarnawski, M. (2015). Assessment of heavy metals mobility and toxicity in contaminated sediments by sequential extraction and a battery of bioassays. Ecotoxicology, 24(6), 1279–1293. https://doi.org/10.1007/s10646-015-1499-4
Bell, F. G., Bullock, S. E. T., Halbich, T. F. J., & Lindsay, P. (2001). Environmental impacts associated with an abandoned mine in the Witbank Coalfield, South Africa. International Journal of Coal Geology, 45(2-3), 195–216. https://doi.org/10.1016/S0166-5162(00)00033-1
Ben-Dor, E., Irons, J. R., & Epema, G. F. (1999). Soil reflectance. In A. N. Rencz (Ed.), Remote Sensing for the Earth Sciences: Manual of Remote Sensing (pp. 111–188). John Wiley & Sons.
Bishop, J.L. (2020). Visible and near-infrared reflectance spectroscopy: Laboratory spectra of geologic materials, In: Bishop, J.L., Bell, J.F., & Moersch, J.E. (Eds.), Remote Compositional Analysis: Techniques for Understanding Spectroscopy, Mineralogy, and Geochemistry of Planetary Surfaces, 68–101.
Bradshaw, A. (2000). The use of natural processes in reclamation – Advantages and difficulties. Landscape Urban Planning, 51, 89–100.
Buccianti, A., & Pawlowsky-Glahn, V. (2005). New perspectives on water chemistry and compositional data analysis. Mathematical Geology, 37(7), 703–727.
Buhrke, V. E., Jenkins, R., & Smith, D. K. (1998). A practical guide for the preparation of specimens for x-ray fluorescence and x-ray diffraction analysis. Wiley-VCH.
Calude, C. S., & Longo, G. (2017). The deluge of spurious correlations in big data. Foundations of Science, 22(3), 595–612. https://doi.org/10.1007/s10699-016-9489-4
Choe, E., van der Meer, F., van Ruitenbeek, F., van der Werff, H., de Smeth, B., & Kim, K. W. (2008). Mapping of heavy metal pollution in stream sediments using combined geochemistry, field spectroscopy, and hyperspectral remote sensing: A case study of the Rodalquilar mining area, SE Spain. Remote Sensing of Environment, 112(7), 3222–3233. https://doi.org/10.1016/j.rse.2008.03.017
Choe, E., Kim, K. W., Bang, S., Yoon, I. H., & Lee, K. Y. (2009). Qualitative analysis and mapping of heavy metals in an abandoned Au-Ag mine area using NIR spectroscopy. Environmental Geology, 58(3), 477–482. https://doi.org/10.1007/s00254-008-1520-9
Chow, G. C. (1960). Tests of equality between sets of coefficients in two linear regressions. Econometrica, 28(3), 591–605.
Clark, R. N. (1999). Spectroscopy of rocks and minerals, and principles of spectroscopy. In A. Rencz (Ed.), Manual of Remote Sensing. John Wiley and Sons Inc..
Clevers, J. G. P. W., Kooistra, L., & Schaepman, M. E. (2008). International Journal of Applied Earth Observation and Geoinformation using spectral information from the NIR water absorption features for the retrieval of canopy water content. International Journal of Applied Earth Observations and Geoinformation, 10(3), 388–397. https://doi.org/10.1016/j.jag.2008.03.003
Covelo, E. F., Vega, F. A., & Andrade, M. L. (2007). Competitive sorption and desorption of heavy metals by individual soil components. Journal of Hazardous Materials, 140, 308–315.
Davis, J. C. (2002). Statistics and data analysis in geology (p. 638). John Wiley & Sons Inc..
de Winter, J.C.F. (2013) Using the student’s t-test with extremely small sample sizes, practical assessment, research, and evaluation: Vol. 18, Article 10. https://doi.org/10.7275/e4r6-dj05
Dragović, S., Howard, B. J., Caborn, J. A., Barnett, C. L., & Mihailović, N. (2010). Transfer of natural and anthropogenic radionuclides to ants, bryophytes and lichen in a semi-natural ecosystem. Environmental Monitoring and Assessment, 166(1–4), 677–686. https://doi.org/10.1007/s10661-009-1032-4
Eby, G.N. (2004). Acid mine drainage, In Dodson, K. (Ed.): Principles of environmental geochemistry, Brookes/Cole, 352–358.
España, J. S. (2007). The behavior of iron and aluminum in acid mine drainage: Speciation, mineralogy, and environmental significance. In: Letcher, T.M (Ed.), Thermodynamics, Solubility and Environmental Issues, Elsevier, p137–150. https://doi.org/10.1016/B978-044452707-3/50009-4
Förstner, U., & Salomons, W. (1980). Trace metal analysis on polluted sediments: Assessment of sources and intensities. Environmental Technology Letters, 1(248), 494.
Frost, J. (2023a). Statistics by Jim. http://statisticsbyjim.com/regression/standard-error-regression-vs-r-squared/. Accessed July 2023
Frost, J. (2023b). Statistics by Jim. https://statisticsbyjim.com/regression/root-mean-square-error-rmse/. Accessed July 2023
Gholizadeh, A., Borůvka, L., Vašát, R., Saberioon, M. M., Klement, A., Kratina, J., Tejnecký, V., & Drábek, O. (2015). Estimation of potentially toxic elements contamination in anthropogenic soils on a brown coal mining dumpsite using reflectance spectroscopy: A case study. PLoS One, 10, e0117457.
Goetz, A. F. H. (2012). Making accurate field spectral measurements. Boulder, CO: ASD Inc., Panalytical company.
Goldberg, S. (2002). Competitive adsorption of arsenate and arsenite on oxides and clay minerals. Soil Science Society of America Journal, 66, 413–421.
González-Costa, J. J., Reigosa, M. J., Matıás, J. M., & Fernández-Covelo, E. (2017). Analysis of the importance of oxides and clays in Cd, Cr, Cu, Ni, Pb and Zn adsorption and retention with regression Trees. PLoS One, 12(1), e0168523. https://doi.org/10.1371/journal.pone.0168523
Grillini, F., Thomas, J. B., & George, S. (2021). Radiometric spectral fusion of VNIR and SWIR hyperspectral cameras, 29th Color and Imaging Conference Final Program and Proceedings. Society for Imaging Science and Technology, 276–281. https://doi.org/10.2352/issn.2169-2629.2021.29.276
Halter, W. E., & Pfeifer, H. R. (2001). Arsenic(V) adsorption onto α-Al2O3 between 25 and 70°C. Applied Geochemistry, 16, 793–802.
Harrell, F. E., Jr. (2001). Regression modeling strategies. Springer-Verlag.
Hunt, G. R., & Ashley, P. (1979). Spectra of altered rocks in the visible and near infrared. Economic Geology, 74, 1613–1629. https://doi.org/10.2113/gsecongeo.74.7.1613
INAP (International Network for Acid Prevention). (2009). The global acid rock drainage guide. Available at http://www.gardguide.com. Accessed Mar 2023
Janse, P. V., Kayte, J. N., Agrawal, R. V., & Deshmukh, R. R. (2018). Standard spectral reflectance measurements for ASD fieldSpec spectroradiometer. PDGC 2018 - 2018 5th International Conference on Parallel, Distributed and Grid Computing, 729–733. https://doi.org/10.1109/PDGC.2018.8745808
Ji, J., Song, Y., Yuan, X., & Yang, Z. (2010). Diffuse reflectance spectroscopy study of heavy metals in agricultural soils of the Changjiand River Delta, China. World, 47–50.
Jiang, Q., Liu, M., Wang, J., & Liu, F. (2018). Feasibility of using visible and near-infrared reflectance spectroscopy to monitor heavy metal contaminants in urban lake sediment. Catena, 162, 72–79. https://doi.org/10.1016/j.catena.2017.11.020
Kabata-Pendias, A., & Pendias, H. (2000). Trace elements is soils and plants. CRC Press.
Kemper, T., & Sommer, S. (2002). Estimate of heavy metal contamination in soils after a mining accident using reflectance spectroscopy. Environmental Science and Technology, 36, 2742–2747.
Kemper, T., Sommer, S., (2003). Mapping and monitoring of residual heavy metal contamination and acidification risk after the Aznalcóllar mining accident (Andalusia, Spain) using field and airborne hyperspectral data. In: Habermeyer, M., Müller, A., Holzwarth, S. (Eds.) Proceedings of 3rd EARSEL Imaging Spectroscopy, Herrsching, Germany, CD-ROM ISBN 2–908885–56–5, 333–343.
Khunsa, F., Muhammad, U. K. K., Allah, B. K., Muhammad, T., Naghma, H., & Asid, U. R. (2017). Minerals identification and mapping using ASTER satellite image. Journal of Applied Remote Sensing, 11(4), 046006. https://doi.org/10.1117/1.JRS.11.046006
Kumpiene, J., Lagerkvist, A., & Maurice, C. (2008). Stabilization of As, Cr, Cu, Pb and Zn in soil using amendments - A review. Waste Management, 28(1), 215–225. https://doi.org/10.1016/j.wasman.2006.12.012
Lamine, S., Petropoulos, G. P., Brewer, P. A., Bachari, N. E. I., Srivastava, P. K., Manevski, K., Kalaitzidis, C., & Macklin, M. G. (2019). Heavy metal soil contamination detection using combined geochemistry and field spectroradiometry in the United Kingdom. Sensors (Switzerland), 19(4). https://doi.org/10.3390/s19040762
Lilliesand, T.M, Kiefer, R.W., & Chipman, J.W. (2015). Remote Sensing and Image Interpretation. 7th Edition. In Photogrammetric Engineering & Remote Sensing 81(8). https://doi.org/10.14358/pers.81.8.615
Malley, D., & Williams, P. (1997). Use of near-infrared reflectance spectroscopy in prediction of heavy metals in freshwater sediment by their association with organic matter. Environmental Science & Technology, 31, 3461–3467.
McCarthy, T. S., & Humphries, M. S. (2012). Contamination of the water supply to the town of Carolina, Mpumalanga. South African Journal of Science, 109(9/10), 10. https://doi.org/10.1590/sajs.2013/20120112
Moreno, A. M., Quintana, J. R., Pérez, L., & Parra, J. G. (2006). Factors influencing lead sorption–desorption at variable added metal concentrations in Rhodoxeralfs. Chemosphere, 64(5), 758–763.
Mustard, J.F. & Glotch T.D. (2020). Theory of reflectance and emittance spectroscopy of geologic materials in the visible and infrared regions, In: Bishop, J.L., Bell, J.F., & Moersch, J.E. (Eds.), Remote Compositional Analysis: Techniques for Understanding Spectroscopy, Mineralogy, and Geochemistry of Planetary Surfaces, 21–41.
Mutiyar, P. K., & Mittal, A. K. (2014). Occurrences and fate of selected human antibiotics in influents and effluents of sewage treatment plant and effluent-receiving river Yamuna in Delhi (India). Environmental Monitoring and Assessment, 186(1), 541–557. https://doi.org/10.1007/s10661-013-3398-6
N’Guessan, Y. M., Probst, J. L., Bur, T., & Probst, A. (2009). Trace elements in stream bed sediments from agricultural catchments (Gascogne region, S-W France): Where do they come from? Science of the Total Environment, 407, 2939–2952.
Netshitungulwana, R., Yibas, B., Novhe, O., & Motlakeng T. (2013). Stream sediment geochemistry of the areas impacted by mining around Emalahleni (Witbank), South Africa: Finger printing AMD potential point sources. . IMWA 2013 - Reliable Mine Water Technology for Sustainable Global Mining. Golden, Colorado, USA, 17–22.
Nieto, J. M., Sarmiento, A. M., Olías, M., Canovas, C. R., Riba, I., Kalman, J., & Delvalls, T. A. (2007). Acid mine drainage pollution in the Tinto and Odiel rivers (Iberian Pyrite Belt, SW Spain) and bioavailability of the transported metals to the Huelva Estuary. Environment International, 33(4), 445–455. https://doi.org/10.1016/j.envint.2006.11.010
Omran, E. S. E. (2016). Inference model to predict heavy metals of Bahr El Baqar soils, Egypt using spectroscopy and chemometrics technique. Modeling Earth Systems and Environment, 2(4), 1–17. https://doi.org/10.1007/s40808-016-0259-7
Osei, B. A., & Singh, B. (2000). Effect of selective removal of organic matter and iron oxides on the specific areas of some tropical soil clays. Ghana Journal of Agricultural Science, 33, 55–61. https://doi.org/10.4314/gjas.v33i1.1884
Palansooriya, K. N., Shaheen, S. M., Chen, S. S., Tsang, D. C. W., Hashimoto, Y., Hou, D., Bolan, N. S., Rinklebe, J., & Ok, Y. S. (2020). Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environment International, 134, 105046. https://doi.org/10.1016/j.envint.2019.105046
Pandit, C. M., Filippelli, G. M., & Li, L. (2010). Estimation of heavy-metal contamination in soil using reflectance spectroscopy and partial least-squares regression. International Journal of Remote Sensing, 31(15), 4111–4123.
Parker, S. R., Gammons, C. H., Jones, C. A., & Nimick, D. A. (2007). Role of hydrous iron oxide formation in attenuation and diel cycling of dissolved trace metals in a stream affected by acid rock drainage. 247–263. https://doi.org/10.1007/s11270-006-9297-5
Pawlowsky-Glahn, V., & Egozcue, J. J. (2006). Compositional data and their analysis. In A. Buccianti, G. Mateu-Figueras, & V. Pawlowsky-Glahn (Eds.), Compositional Data Analysis in the Geosciences: From Theory to Practice: Special Publications (Vol. 264, pp. 1–10). Geological Society.
Piroozfar, P., Alipour, S., Modabberi, S., & Cohen, D. (2018). Application of geochemistry and VNIR spectroscopy in mapping heavy metal pollution of stream sediments in the Takab mining area of Iran. Acta Geologica Sinica (English Edition), 92(6), 2382–2394.
Poppelars, J. (2015). OR at work. http://john-poppelaars.blogspot.fr/2015/04/do-numbers-really-speak-for-themselves.html. Accessed July 2023
Prasad, K. A., & Gnanappazham, L. (2016). Multiple statistical approaches for the discrimination of mangrove species of Rhizophoraceae using transformed field and laboratory hyperspectral data. Geocarto International, 31(8), 891–912. https://doi.org/10.1080/10106049.2015.1094521
Prasad, K. A., Gnanappazham, L., Selvam, V., Ramasubramanian, R., & Kar, C. S. (2015). Developing a spectral library of mangrove species of Indian east coast using field spectroscopy. Geocarto International, 30(5), 580–599. https://doi.org/10.1080/10106049.2014.985743
Ramsey, M. H. (1998). Sampling as a source of measurement uncertainty: Techniques for quantification and comparison with analytical sources. Journal of Analytical Atomic Spectrometry, 13, 97–104. https://doi.org/10.1039/A706815H
Reimann, C., & de Caritat, P. (2017). Establishing geochemical background variation and threshold values for 59 elements in Australian surface soil. Science of the Total Environment, 578, 633–648. https://doi.org/10.1016/j.scitotenv.2016.11.010
Reimann, C., & Filzmoser, P. (2000). Normal and lognormal data distribution in geochemistry: Death of a myth. Consequences for the statistical treatment of geochemical and environmental data. Environmental Geology, 39(9), 1001–1014.
Ren, H., Zhuang, D. F., & Sing, A. N. (2009). Estimation of As and Cu contamination in agricultural soils around a mining area by reflectance spectroscopy: A case study. Pedosphere, 19, 719–726.
Sawut, R., Kasim, N., Abliz, A., Hu, L., Yalkun, A., Maihemuti, B., & Qingdong, S. (2018). Possibility of optimized indices for the assessment of heavy metal contents in soil around an open pit coal mine area. International Journal of Applied Earth Observation and Geoinformation, 73, 14–25. https://doi.org/10.1016/j.jag.2018.05.018
Schindler, P. W., & Sposito, G. (1991). Surface complexation at (hydr)oxide surfaces. In G. H. Bolt et al. (Eds.), Interactions at the Soil Colloid-Soil Solution Interface (p. 115). NATO ASI Sen, Kluwer Academic.
Sengupta, M. (1993). Environmental impacts of mining: Monitoring, restoration, and control (p. 351). Lewis Publishers.
Shahbazi, K., & Beheshti, M. (2019). Comparison of three methods for measuring heavy metals in calcareous soils of Iran. SN Applied Sciences, 1(12), 1–19. https://doi.org/10.1007/s42452-019-1578-x
Song, L., Jian, J., Tan, D.-J., Xie, H. B., Luo, Z. F., & Gao, B. (2015). Estimate of heavy metals in soil and streams using combined geochemistry and field spectroscopy in Wan-sheng mining area, Chongqing, China. International Journal of Applied Earth Observation and Geoinformation, 34, 1–9.
Sotirakopoulos, K., Barham, R., Piper, B., & Nencini, L. (2015). A statistical method for assessing network stability using the Chow test. Environmental Sciences: Processes and Impacts, 17(10), 1841–1850. https://doi.org/10.1039/c5em00325c
Steyerberg, E. W. (2009). Clinical prediction models. Springer-Verlag.
Tu, Y., Zou, B., Feng, H., Zhou, M., Yang, Z., & Xiong, Y. (2021). A near standard soil samples spectra enhanced modeling strategy for cd concentration prediction. Remote Sensing, 13(14). https://doi.org/10.3390/rs13142657
Uddin, M. K. (2017). A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chemical Engineering Journal, 308, 438–462. https://doi.org/10.1016/j.cej.2016.09.029
Ugwu, I. M., & Igbokwe, O. A. (2019). Sorption of heavy metals on clay minerals and oxides: A review. In S. Edebali (Ed.), Advanced Sorption Process Applications. IntechOpen.
Van der Meer, F. (1999). Can we map swelling clay with remote sensing? International Journal of Applied Earth Observation and Geoinformation, 1, 27–35.
Van der Meer, F. (2004). Analysis of spectral absorption features in hyperspectral imagery. International Journal of Applied Earth Observation and Geoinformation, 5(1), 55–68. https://doi.org/10.1016/j.jag.2003.09.001
Van der Meer, F., & Jia, X. (2012). Collinearity and orthogonality of endmembers in linear spectral unmixing. International Journal of Applied Earth Observation and Geoinformation, 18, 491–503.
Van Voorhis, C. W., & Morgan, B. L. (2007). Understanding power and rules of thumb for determining sample sizes. Tutorials in Quantitative Methods for Psychology, 3(2), 43–50.
Walling, D. E., & He, Q. (1998). The spatial variability of overbank sedimentation on river floodplains. Geomorphology, 24(2-3), 209–223.
Wang, Y., Zhao, Y., & Xu, S. (2022). Application of VNIR and machine learning technologies to predict heavy metals in soil and pollution indices in mining areas. Journal of Soils and Sediments, 22(10), 2777–2791. https://doi.org/10.1007/s11368-022-03263-3
Webster, J. G., Swedlund, P. J., & Webster, K. S. (1998). Trace metal adsorption onto an acid mine drainage iron(III) oxy hydroxy sulfate. Environmental Science and Technology, 32(10), 1361–1368. https://doi.org/10.1021/es9704390
Wu, Y., Chen, J., Ji, J., Gong, P., Liao, Q., Tian, Q., & Ma, H. (2007). A mechanism study of reflectance spectroscopy for investigating heavy metals in soils. Soil Science Society of America Journal, 71(3), 918–926.
Wu, Y. Z., Chen, J., Ji, J. F., Tian, Q. J., & Wu, X. M. (2005). Feasibility of reflectance spectroscopy for the assessment of soil mercury contamination. Environmental Science and Technology, 39(3), 873–878. https://doi.org/10.1021/es0492642
Xie, X., Pan, X. Z., & Sun, B. (2012). Visible and near-infrared diffuse reflectance spectroscopy for prediction of soil properties near a copper smelter. Pedosphere, 22, 351–366.
Yadav, S., & Shukla, S. (2016). Analysis of k-fold cross-validation over hold-out validation on colossal datasets for quality classification, 2016 IEEE 6th International Conference on Advanced Computing (IACC), 78–83. https://doi.org/10.1109/IACC.2016.25
Zhang, S., Chen, Y., Zhang, Z., Wang, S., Wu, Z., Hong, Y., Wang, Y., Hou, H., Hu, Z., & Fei, T. (2022). VNIR estimation of heavy metals concentrations in suburban soil with multi-scale geographically weighted regression. Catena, 219, 106585. https://doi.org/10.1016/j.catena.2022.106585
Zhang, X., Sun, W., Cen, Y., Zhang, L., & Wang, N. (2019). Predicting cadmium concentration in soils using laboratory and field reflectance spectroscopy. Science of the Total Environment, 650, 321–334. https://doi.org/10.1016/j.scitotenv.2018.08.442
Zhao, L., Hong, H., Fang, Q., Algeo, T. J., Wang, C., Li, M., & Yin, K. (2020). Potential of VNIR spectroscopy for prediction of clay mineralogy and magnetic properties, and its paleoclimatic application to two contrasting Quaternary soil deposits. Catena, 184, 104239. https://doi.org/10.1016/j.catena.2019.104239
Zhuang, J., & Yu, G. R. (2002). Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere, 49(6), 619–628. https://doi.org/10.1016/S0045-6535(02)00332-6
Zief, M., & Mitchell, J. W. (1976). Contamination control in trace metals analysis. Chemical Analysis, 47(Chapter 6).
Funding
Open access funding provided by University of the Free State. Funding for this research was provided by the South African National Space Agency (SANSA). SANSA has had no involvement in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
Jamie-Leigh Robin Abrahams contributed to the conceptualization, data collection, formal analysis, investigation, visualization, writing—original draft and Emmanuel John Muico Carranza contributed to the conceptualization, data collection, supervision, resources, writing—review and editing.
Corresponding author
Ethics declarations
Ethics approval
All authors have read, understood, and have complied as applicable with the statement on “Ethical responsibilities of Authors” as found in the Instructions for Authors.
Consent for publication
All the authors have given their consent for the publication of this article and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
bThe number refers to the absorption-band position, namely the wavelength corresponding with the minimum reflectance percentage within the wavelength range of the absorption feature (Van der Meer, 2004).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abrahams, JL.R., Carranza, E.J.M. Trace metal content prediction along an AMD (acid mine drainage)-contaminated stream draining a coal mine using VNIR–SWIR spectroscopy. Environ Monit Assess 195, 1261 (2023). https://doi.org/10.1007/s10661-023-11837-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-023-11837-y