Abstract
During a survey in 2011–2012, three ornamental plants of Araceae namely Aglaonema nitidum, Syngonium podophyllum and Dieffenbachia amoena showing foliar disease symptoms were collected from central region of Iran. Infected plants exhibited spots on their leaves which appeared as yellow and water-soaked with chlorotic haloes and necrotic center. To investigate the etiology of this disorder, symptomatic leaves were collected from affected plants and six bacterial strains (B2Y, J3Y, SY, E60Y, E68Y and E5MM) were isolated and identified as Pantoea ananatis or P. agglomerans based on morphological, physiological, biochemical and molecular characters. The pathogenicity tests of the isolates demonstrated that they were not host specific. Furthermore, 16S rRNA gene sequencing revealed that the strains were phylogenetically closely related to genus Pantoea. Multilocus sequence analysis (MLSA) of concatenated partial atpD, gyrB and rpoB gene sequences of the six isolates showed a high similarity of B2Y, J3Y, and SY strains to P. ananatis and also of E60Y, E5MM and E68Y strains to P. agglomerans. These results were confirmed by phylogenetic analysis. To the best of our knowledge, this is the first report of leaf spot and necrosis of A. nitidum, S. podophyllum and D. amoena caused by the genus Pantoea.






Similar content being viewed by others
References
Agrios, G. N. (2005). Plant Pathology (5th ed.). New York: Academic Press.
Alippi, A. M., & Lopez, A. C. (2009). First Report of Pectobacterium carotovorum subsp. carotovorum on Spathiphyllum wallisii in Argentina. Plant Disease, 93, 842.
Azad, H. R., Holmes, G. J., & Cooksey, D. A. (2000). A new leaf blotch disease of sudangrass caused by Pantoea ananas and Pantoea stewartii. Plant Disease, 84, 973–979.
Baghaee-Ravari, S., Rahimian, H., Shams-Bakhsh, M., Lopez-Solanilla, E., Antúnez-Lamas, M., & Rodríguez-Palenzuela, P. (2011). Characterization of Pectobacterium species from Iran using biochemical and molecular methods. European Journal of Plant Pathology, 129, 413–425.
Barash, I., & Manulis-Sasson, S. (2009). Recent evolution of bacterial pathogens: The gall-forming Pantoea agglomerans case. Annual Review of Phytopathology, 47, 133–152.
Brady, C. L., Cleenwerck, I., Venter, S. N., Vancanneyt, M., Swings, J., & Coutinho, T. A. (2008). Phylogeny and identification of Pantoea species associated with plants, humans and the natural environment based on multilocus sequence analysis (MLSA). Systematic and Applied Microbiology, 31, 447–460.
Brady, C. L., Venter, S. N., Cleenwerck, I., Engelbeen, K., Vancanneyt, M., Swings, .J., & Coutinho, T. A. (2009). Pantoea vagans sp. nov., Pantoea eucalypti sp. nov., Pantoea deleyi sp. nov. and Pantoea anthophila sp. nov. International Journal of Systematic and Evolutionary Microbiology, 59, 2339–2345.
Brady, C. L., Cleenwerck, I., Venter, S. N., Engelbeen, K., de Vos, P., & Coutinho, T. A. (2010). Emended description of the genus Pantoea and description of four novel species from human clinical samples, Pantoea septica sp. nov., Pantoea eucrina sp. nov., Pantoea brenneri sp. nov. and Pantoea conspicua sp. nov., and transfer of Pectobacterium cypripedii (Hori 1911) Brenner et al. 1973 emend. Hauben et al. 1998 to the genus Pantoea emend. as Pantoea cypripedii comb. nov. International Journal of Systematic and Evolutionary Microbiology, 60, 2430–2440.
Brady, C. L., Goszczynska, T., Venter, S. N., Cleenwerck, I., De Vos, P., Gitaitis, R. D., & Coutinho, T. A. (2011). Pantoea allii sp. nov., a novel species isolated from onion and onion seed. International Journal of Systematic and Evolutionary Microbiology, 61, 932–937.
Brady, C. L., Cleenwerck, I., van der Westhuizen, L., Venter, S. N., Coutinho, T. A., & De Vos, P. (2012). Pantoea rodasii sp. nov., Pantoea rwandensis sp. nov. and Pantoea wallisii sp. nov., isolated from Eucalyptus. International Journal of Systematic and Evolutionary Microbiology, 62, 1457–1464.
Bruton, B. D., Wells, J. M., Lester, G. E., & Patterson, C. L. (1991). Pathogenicity and characterization of Erwinia ananas causing a postharvest disease of cantaloupe fruit. Plant Disease, 75, 180–183.
Chase, A. R. (1987). Compendium of ornamental foliage plant diseases (no. 635.923/C487). St. Paul: APS press.
Cilia, V., Lafay, B., & Christen, R. (1996). Sequence heterogeneities among 16s ribosomal RNA sequences, and their effect on phylogenetic analyses at the species level. Molecular Biology and Evolution, 13(3), 451–461.
Clarridge, J. E. (2004). Impact of 16SrRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clinical Microbiology Reviews, 17, 840–862.
Coplin, D. L., & Kado, C. I. (2001). Gram-negative bacteria Pantoea. In N. W. Schaad (Ed.), Laboratory guide for identification of plant pathogenic bacteria (pp. 73–83). St. Paul: APS Press.
Cortesi, P., & Pizzatti, C. (2007). Palea browning, a new disease of rice in Italy caused by Pantoea ananatis. Journal of Phytopathology, 89, S76.
Coutinho, T. A., & Venter, S. N. (2009). Pantoea ananatis: An unconventional plant pathogen. Molecular Plant Pathology, 10(3), 325–335.
Coutinho, T. A., Preisig, O., Mergaert, J., Cnockaert, M. C., Riedel, K. H., Swings, J., & Wingfield, M. J. (2002). Bacterial blight and dieback of Eucalyptus species, hybrids, and clones in South Africa. Plant Disease, 86, 20–25.
De Baere, T., Verhelst, R., Labit, C., Verschraegen, G., Wauters, G., Claeys, G., & Vaneechoutte, M. (2004). Bacteremic infection with Pantoea ananatis. Journal of Clinical Microbiology, 42, 4393–4395.
Deletoile, A., Decré, D., Courant, S., Passet, V., Audo, J., Grimont, P., Arlet, G., & Brisse. (2009). Phylogeny and identification of Pantoea species and typing of Pantoea agglomerans strains by multilocus gene sequencing. Journal of Clinical Microbiology, 47, 300–310.
Frederickson, D. E., Monyo, E. S., King, S. B., & Odvody, G. N. (1997). A disease of pearl millet in Zimbabwe caused by Pantoea agglomerans. Plant Disease, 81, 959–964.
Fucikovsky, L., & Aranda, S. (2006). Pantoea ananatis a new pathogen of agave in Mexico. Phytopathology, 96, S37.
Gardan, L., Dauga, C., Prior, P., Gillis, M., & Saddler, G. S. (2000). Acidovorax anthurii sp. nov., a new phytopathogenic bacterium which causes bacterial leaf-spot of anthurium. International Journal of Systematic and Evolutionary Microbiology, 50, 235–246.
Gavini, F., Mergaert, J., Beji, A., Mielcarek, C., Izard, D., Kersters, K., & De Ley, J. (1989). Trasfer of Entrobacter agglomerans (Beijerinck 1888) Ewing and fife 1972 to Pantoea gen. Nov. as Pantoea agglomerans comb. Nov. and description of Pantoea dispersa sp. Nov. International Journal of Systematic Bacteriology, 39, 337–345.
Gitaitis, R. D., Walcott, R., Culpepper, S., Sanders, H., Zolobowska, L., & Langston, D. (2002). Recovery of Pantoea ananatis, causal agent of center rot of onion, from weeds and crops in Georgia, USA. Crop Protection, 21, 983–989.
Goszczynska, T., Botha, W. J., Venter, S. N., & Coutinho, T. A. (2007). Isolation and identification of the causal agent of brown stalk rot, a new disease of maize in South Africa. Plant Disease, 91, 711–718.
Grimont, P. A. D., & Grimont, F. (2005). Genus XXIII. Pantoea. Bergey’s Manual of Systematic Bacteriology, 2, 713–720.
Gueule, D., Fourny, G., Ageron, E., Le Flèche-Matéos, A., Vandenbogaert, M., Grimont, P. A., & Cilas, C. (2015). Pantoea coffeiphila sp. nov., cause of the 'potato taste' of Arabica coffee from the African Great Lakes region. International Journal of Systematic and Evolutionary Microbiology, 65, 23–29.
Hauben, L., Vauterin, L., Swings, J., & Moore, E. R. B. (1997). Comparison of 16S ribosomal DNA sequences of all Xanthomonas species. International Journal of Systematic Bacteriology, 47(2), 328–335.
Krawczyk, K., Kamasa, J., Zwolinska, A., & Pospieszny, H. (2010). First report of Pantoea ananatis associated with leaf spot disease of maize in Poland. Journal of Plant Pathology, 92(3), 807–811.
Larget, B., & Simon, D. L. (1999). Markov chain Monte Carlo algorithms for the Bayesian analysis of phylogenetic trees. Molecular Biology and Evolution, 16, 750–759.
Lelliott, R., Billing, E., & Hayward, A. (1966). A determinative scheme for the fluorescent plant pathogenic pseudomonads. Journal of Applied Microbiology, 29(3), 470–489.
Lindow, S. E., Desurmont, C., Elkins, R., McGourty, G., Clark, E., & Brandl, M. T. (1998). Occurrence of indole-3 acetic acid-producing bacteria on pear trees and their association with fruit russet. Phytopathology, 88, 1149–1157.
Manulis, S., & Barash, I. (2003). Pantoea agglomerans pvs. gypsophilae and betae, recently evolved pathogens. Molecular Plant Pathology, 4, 307–314.
McFadden, L. (1962). Two bacterial pathogens affecting leaves of Aglaonema robelinii. Phytopathology, 52, 20.
Medrano, E. G., & Bell, A. A. (2007). Role of Pantoea agglomerans in opportunistic bacterial seed and ball rot of cotton (Gossypium hirsutum) grown in the field. Journal of Applied Microbiology, 102, 134–143.
Mergaert, J., Verdonck, L., & Kersters, K. (1993). Transfer of Erwinia ananas (synonym, Erwinia uredovora) and Erwinia stewartii to the genus Pantoea emend as Pantoea ananas (serrano 1928) comb. Nov. and Pantoea stewartii (smith 1898) comb. Nov., respectively, and description of Pantoea stewartii subsp. indologenes subsp. nov. International Journal of Systematic Bacteriology, 43, 162–173.
Noller, H. F., Hong, L., & Fredrick, K. (2005). The 30s ribosomal P site: A function of 16SrRNA. FEBS Letters, 579, 855–858.
Nylander, J. A. A. (2004). MrModeltest Version 2. Program distributed by the author. Uppsala: Evolutionary Biology Centre: Uppsala University.
Paccola-Meirelles, L. D., Ferreira, A. S., Meirelles, W. F., Marriel, I. E., & Casela, C. R. (2001). Detection of a bacterium associated with a leaf spot disease of maize in Brazil. Journal of Phytopathology, 149, 275–279.
Perombelon, M. C. M., & Hyman, L. J. (1986). A rapid method for identifying and quantifying soft rot erwinias directly from plant material based on their temperature tolerance and sensitivity to erythromycin. Journal of Applied Bacteriology, 60, 61–66.
Perombelon, M. C. M., & Van der Wolf, J. M. (2002). Methods for the detection and quantification of Erwinia carotovora subsp. atroseptica (Pectobacterium carotovorum subsp. atrosepticum) on potatoes: A laboratory manual. Dundee: Scottish Crop research institute occasional publication no. 10.
Popp, A., Cleenwerck, I., Iversen, C., De Vos, F., & Stephan, R. (2010). Pantoea gaviniae sp. nov. and Pantoea calida sp. nov., isolated from infant formula and an infant formula production environment. International Journal of Systematic and Evolutionary Microbiology, 60, 2786–2792.
Pusey, P. L., Stockwell, V. O., Reardon, C. L., Smits, T. H. M., & Duffy, B. (2011). Antibiosis activity of Pantoea agglomerans biocontrol strain E325 against Erwinia amylovora on apple flower stigmas. Phytopathology, 101, 1234–1241.
Romeiro, R. S., Macagnan, D., Mendonca, H. L., & Rodrigues Neto, J. (2006). Bacterial spot of Chinese taro (Alocasia cucullata) in Brazil induced by Pantoea agglomerans. New Disease Reports, 14, 51.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Schaad, N. W., Jones, J. B., & Chun, W. (2001). Laboratory guide for identification of plant pathogenic bacteria (3rd ed.). St. Paul: APS: The American Phytopathological Society.
Sherafati, F., Khodaygan, P., Azadvar, M., Sedaghati, E., Saberi-Riseh, R., & Baghaee-Ravari., S. (2014). Association of Pantoea agglomerans with the citrus bacterial canker disease in Iran. Journal of Crop Protection, 3 (3), 345–355.
Stephan, R., Van Trappen, S., Cleenwerck, I., Vancanneyt, M., De Vos, P., & Lehner, A. (2007). Enterobacter turicensis sp. nov. and Enterobacter helveticus sp. nov., isolated from fruit powder. International Journal of Systematic and Evolutionary Microbiology, 57, 820–826.
Vantomme, R., Mergaert, J., Verdonck, L., & De Ley, J. (1989). Antagonistic effect in vitro of Erwinia uredovora LMG 2678 against some other bacteria. Journal of Phytopathology, 124, 372–376.
Walcott, R. R., Gitaitis, R. D., Castro, A. C., Sanders Jr., F. H., & Diaz-Perez, J. C. (2002). Natural infestation onion seed by Pantoea ananatis, causal agent of center rot. Plant Disease, 86, 106–111.
Walterson, A. M., & Stavrinides, J. (2015). Pantoea: Insights into a highly versatile and diverse genus within the Enterobacteriaceae. FEMS Microbiology Reviews, 39(6), 968–984.
Weinthal, D. M., Barash, I., Tzfira, T., Gaba, V., Teper, D., Sessa, G., & Manulis-Sasson, S. (2011). Characterization of nuclear localization signals in the type III effectors HsvG and HsvB of the gall-forming bacterium Pantoea agglomerans. Microbiology, 157, 1500–1508.
Yazdani, R., Shams-Bakhsh, M., & Safaie, N. (2015). Identification of bacterial leaf spot and soft rot agent on ornamental plants in Isfahan province. Plant Protection, 38(1), 91–100.
Acknowledgements
We gratefully acknowledge financial support from Tarbiat Modares University, Tehran, Iran.
Author information
Authors and Affiliations
Contributions
Razieh Yazdani carried out all experiments, drafting the manuscript;
Naser Safaie gave advice;
Masoud Shams-bakhsh supervised the project, conception and design of study, edited the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
The result of this work has not been published previously and is not under consideration elsewhere.
Funding
This research was funded by Tarbiat Modares University, Tehran, Iran.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Fig. S1
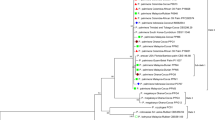
Bayesian 50% majority rule consensus tree inferred from 90 sequences of partial gyrB under the GTR + I + G model. At nodes are given posterior probabilities values >50%. The new sequences are in bold. (JPEG 3333 kb)
Fig. S2
Bayesian 50% majority rule consensus tree inferred from 97 sequences of partial rpoB under the GTR + I + G model. At nodes are given posterior probabilities values >50%. The new sequences are in bold. (JPEG 3604 kb)
Fig. S3
Bayesian 50% majority rule consensus tree inferred from 103 sequences of partial atpD under the GTR + I + G model. At nodes are given posterior probabilities values >50%. The new sequences are in bold. (JPEG 3324 kb)
Rights and permissions
About this article
Cite this article
Yazdani, R., Safaie, N. & Shams-Bakhsh, M. Association of Pantoea ananatis and Pantoea agglomerans with leaf spot disease on ornamental plants of Araceae Family. Eur J Plant Pathol 150, 167–178 (2018). https://doi.org/10.1007/s10658-017-1264-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1264-z