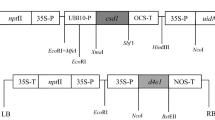
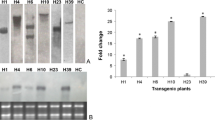
Abstract
Citrus canker, caused by the bacterial pathogen Xanthomonas citri subp. Citri (Xcc), is a serious disease reported in most citrus-producing areas around the world. Although different levels of field resistance to citrus canker have been reported in sweet oranges, they are usually not sufficient to provide adequate control of the disease. Ectopic over-expression of antibacterial genes is one of the potential strategies to increase plant resistance to bacterial diseases. Previous in vitro results showed that sarcotoxin IA, an antimicrobial peptide isolated from the flesh fly (Sarcophaga peregrina), can be efficient to control different plant pathogenic bacteria, including Xcc. Transgenic “Pera” sweet orange (Citrus sinensis [L.] Osbeck) plants constitutively expressing the sarcotoxin IA peptide fused to the PR1a signal peptide from Nicotiana tabacum for secretion in the intercellular space were obtained by Agrobacterium-mediated transformation using thin sections of mature explants. Citrus canker resistance evaluation in leaves of transgenic and non-transgenic plants was performed through inoculations with Xcc by infiltration and spraying. The Xcc population was up to 2 log unit lower in leaves of the transgenic plants compared to those of non-transgenic controls. Incidence of canker lesions was significantly higher in non-transformed controls (>10 lesions/cm2) than in the transgenic plants (<5 lesions/cm2) after injection infiltration or spraying with Xcc inoculum. Accumulation of sarcotoxin IA peptide in sweet orange tissue did not cause any deleterious effects on the growth and development of the transgenic plants, indicating this approach is suitable to provide resistance to citrus canker.







Similar content being viewed by others
References
Behlau, F., Belasque Jr., J., Bergamin, A. F., Graham, J. H., Leite Jr., R. P., & Gottwald, T. R. (2008). Coppersprays and windbreaks for control of citrus canker on young orange trees in southern Brazil. Crop Protection, 27(3–5), 807–813.
Bradford, M. M. (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Bronson, C. H., & Gaskalla, R. (2007). Comprehensive report on citrus canker in Florida. Division of Plant Industry: Florida Department of Agriculture and Consumer Services.
Cardoso, S. C., Barbosa-Mendes, J. M., Boscariol-Camargo, R. L., Christiano, R. S. C., Filho, A. B., Vieira, M. L. C., Mendes, B. M. J., & Mourão Filho, F. A. A. (2010). Transgenic sweet Orange (Citrus sinensis L. Osbeck) expressing the attacin a Gene for resistance to Xanthomonas citri subsp. citri. Plant Molecular Biology Reporter, 28(2), 185–192.
Carvalho, S. A., Nunes, W. M. C., Belasque Jr., J., Machado, M. A., Croce-Filho, J., Bock, C. H., & Abdo, Z. (2015). Comparison of resistance to asiatic citrus canker among different genotypes of citrus in a long-term canker-resistance field screening experiment in Brazil. Plant Disease, 99(2), 207–218.
Cervera, M., Juárez, J., Navarro, A., Pina, J. A., Durán-Vila, N., Navarro, L., & Peña, L. (1998). Genetic transformation and regeneration of mature tissues of woody fruit plants bypassing the juvenile stage. Transgenic Research, 7(1), 51–59.
Da Silva, A. C. R., Ferro, J. A., Reinach, F. C., Farah, C. S., Furlan, L. R., Quaggio, R. B., et al. (2002). Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature, 417, 459–463.
Delaporta, S. L., Wood, J., & Hicks, J. B. (1983). A plant minipreparation: Version II. Plant Molecular Biology Reporter, 4, 19–21.
Düring, K., Porsch, P., Fladung, M., Lõrz, H. (1993). Transgenic potato plants resistant to the phytopathogenic bacterium Erwinia carotovora. The Plant Journal, 3, 587–598.
Dutt, M., Barthe, G., Irey, M., & Grosser, J. (2015). Transgenic citrus expressing an Arabidopsis NPR1 Gene exhibit enhanced resistance against Huanglongbing (HLB; citrus greening). PloS One, 10(9), e0137134. doi:10.1371/journal.pone.0137134.
Furman, N., Kobayashi, K., Zanek, M. C., Calcagno, J., Garcia, M. L., & Mentaberry, A. (2013). Transgenic sweet orange plants expressing a dermaseptin coding sequence show reduced symptoms of citrus canker disease. Journal of Biotechnology, 167, 412–419.
Gmitter Jr., F. G., Grosser, J. W., & Moore, G. A. (1992). Citrus. In F. A. Hammerschlag & R. E. Litz (Eds.), Biotechnology of perennial crops (pp. 335–369). CAB International: Cambridge.
Gottwald, T. R., Graham, J. H., Civerolo, E. L., Barrett, H. C., & Hearn, C. J. (1993). Differential host range reaction of citrus and citrus relatives to citrus canker and citrus bacterial spot determined by leaf mesophyll susceptibility. Plant Disease, 77, 1004–1009.
Gottwald, T. R., Graham, J. H., & Schubert, T. S. (2002). Citrus canker: The pathogen and its impact. Plant Health Progress: Resource document http://plantmanagementnetwork.org/pub/php/review/citruscanker. Accessed 14 Oct 2016.
Gottwald, T., Graham, J., Bock, C., Bonn, G., Civerolo, E., Irey, M., et al. (2009). The epidemiological significance of post-packinghouse survival of Xanthomonas citri ssp. citri for dissemination of Asiatic citrus canker via infected fruit. Crop Protection, 28, 508–524.
Graham, J. H., Gottwald, T. R., Cubero, J., & Achor, D. S. (2004). Xanthomonas axonopodis pv. citri: Factors affecting successful eradication of citrus canker. Molecular Plant Pathology, 5(1), 1–15.
He, Y. R., Chen, S. C., Peng, A. H., Zou, X. P., Xu, L. Z., Lei, T. G., Liu, X. F., & Yao, L. X. (2011). Production and evaluation of transgenic sweet orange (Citrus sinensis L. Osbeck) containing bivalent antibacterial peptide genes (Shiva A and Cecropin B) via a novel Agrobacterium-mediated transformation of mature axillary buds. Scientia Horticulturae, 128, 99–107.
Hao, G., Stover, E., & Gupta, G. (2016). Overexpression of a modified plant thionin enhances disease resistance to citrus canker and Huanglongbing (HLB). Frontiers in Plant Science. doi:10.3389/fpls.2016.01078.
Jaymes, J. M., Nagpala, P., Destéfano-Beltrán, L., Huang, J. H., Kim, J., Denny, T., & Cetiner, S. (1993). Expression of a cecropin B lytic peptide analog in transgenic tobacco confers enhanced resistance to bacterial wilt caused by Pseudomonas solanacearum. Plant Science, 98, 43–53.
Kanai, A., & Natori, S. (1989). Cloning of gene cluster for sarcotoxin I, antibacterial proteins of Sarcophaga peregrina. FEBS Letters, 258(2), 199–202. doi:10.1016/0014-5793(89)81652-7.
Kobayashi, A. K., Bespalhok, J. C., Pereira, L. F. P., & Vieira, L. G. E. (2003). Plant regeneration of sweet orange (Citrus sinensis) from thin sections of mature stem segments. Plant Cell Tissue and Organ Culture, 74(1), 99–102.
Leite, Jr R.P., (1990). Cancro cítrico: prevenção e controle no Paraná. Instituto Agronômico do Paraná, Londrina, p. 51 (IAPAR. Circular, 61).
Lloyd, G.B, & McCown, B.H. (1980). Commercially feasible micropropagation of mountain laurel (Kalmia latifolia) by use of shoot tip culture. Proceedings, international plant propagators, 30: 421-437.
Mitsuhara, I., Matsuura, H., Ohshima, M., Kaku, H., Nakajima, Y., Murai, N., Natori, S., & Ohashi, Y. (2000). Induced expression of sarcotoxin IA enhanced host resistance against both resistance against both bacterial and fungal pathogens in transgenic tobacco. Molecular Plant Microbe Interaction, 13, 860–868.
Mitsuhara, I., Nakajima, Y., Natori, S., Mitsuoka, T., & Ohashi, Y. (2001). In vitro growth inhibition of human intestinal bacteria by sarcotoxin IA, an insect bactericidal peptide. Biotechnology Letters, 23, 569–573.
Mourgues, F., Brisset, M., & Chevreau, E. (1998). Strategies to improve plant resistance to bacterial diseases through genetic engineering. Trends in Biotechnology, 16, 203–210.
Murashige, T., & Skoog, F. (1962). A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum, 15, 473–479.
Nakajima, Y., Qu, X.M. & Natori, S. (1987). Interaction between liposomes and sarcotoxin IA, a potential antibacterial protein of Sarcophaga peregrina (flesh fly). Journal Biological Chemistry, 262, 1665–1669.
Navarro, L. (1992). Citrus shoot-tip grafting in vitro. In Y. P. S. Bajaj (Ed.), Biotechnology in agriculture and forestry (Vol. 18, pp. 328–338). New York: Spring Verlag, Berlin.
Ohshima, M., Mitsuhara, I., Okamoto, M., Sawano, S., Nishiyama, K., Kaku, H., Natori, S., & Ohashi, Y. (1999). Enhanced resistance to bacterial diseases of transgenic tobacco plants overexpressing sarcotoxin IA, a bactericidal peptide of insect. Journal of Biochemistry, 125, 431–435.
Okamoto, M., Mitsuhara, I., Ohshima, M., Natori, S., & Ohashi, Y. (1998). Enhanced expression of an antimicrobial peptide sarcotoxin IA by GUS fusion in transgenic tobacco plants. Plant Cell Physiology, 39, 57–63.
Rodríguez, A., Cervera, M., Peris, J. E., & Peña, L. (2008). The same treatment for transgenic shoot regeneration elicits the opposite effect in mature explants from two closely related sweet orange (Citrus sinensis (L.) Osb.) genotypes. Plant Cell, Tissue and Organ Culture, 93(1), 97–106.
Sambrock, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: A laboratory manual (2nd ed.). Cold Spring Harbor: Cold Spring Harbor Laboratory Press.
Sharma, A., Sharma, R., Imamura, M., Yamakawa, M., & Machii, H. (2000). Transgenic expression of cecropin B, an antibacterial peptide from Bombyx mori, confers enhanced resistance to bacterial leaf blight in rice. FEBS Letters, 484, 7–11.
Stall, R. E., Marcó, G. M., & Echenique, B. I. C. (1982). Importance of mesophyll in mature- leaf resistance to cancrosis of citrus. Phytopathology, 72, 1097–1100.
Viloria, Z., Drouillard, D. L., Graham, J. H., & Grosser, J. W. (2004). Screening triploid hybrids of ‘Lakeland’ limequat for resistance to citrus canker. Plant Disease, 88, 1056–1060.
Yamada, K., Nakajima, Y., & Natori, S. (1990). Production of recombinant sarcotoxin IA in Bombyx mori cells. Biochemistry Jornal, 272, 633–636.
Zhang, X., Francis, M. I., Dawson, W. O., Graham, J. H., Orbović, V., Triplett, E. W., & Mou, Z. (2010). Over-expression of the Arabidopsis NPR1 gene in citrus increases resistance to citrus canker. European Journal of Plant Pathology, 128, 91–100.
Wally, O., & Punja, Z. K. (2010). Genetic engineering for increasing fungal and bacterial disease resistance in crop plants. GM Crops, 1, 199–206.
Acknowledgements
The authors gratefully thanks to Dr. Yuko Ohashi (Plant-Microbe Interactions Research Unit, National Institute of Agrobiological Sciences, Tsukuba, Ibaraki, Japan) for providing the pST10 plasmid. We also thank Suely A. Kudo and Luciana Meneguin for technical assistance. We thank Dr. Nelson A. Wulff, Dr. Leandro Peña and Dr. James Graham for critical reading of this manuscript. This work received financial support from CNPq, Fundação Araucária and Fundo de Defesa da Citricultura – FUNDECITRUS. L. G. E. Vieira and L. F. P. Pereira are CNPq research fellows.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kobayashi, A.K., Vieira, L.G.E., Bespalhok Filho, J.C. et al. Enhanced resistance to citrus canker in transgenic sweet orange expressing the sarcotoxin IA gene. Eur J Plant Pathol 149, 865–873 (2017). https://doi.org/10.1007/s10658-017-1234-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-017-1234-5