Abstract
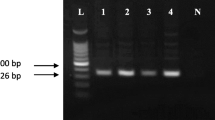
Two different molecular tools for the diagnosis of the cereal and legume root-lesion nematode Pratylenchus thornei were developed. A randomly amplified DNA (RAPD) fragment specific to P. thornei was identified. After sequencing the fragment, longer primers were designed that complement the terminal sequences of the RAPD fragment, and this pair of specific primers was used to amplify the sequence-characterized amplified region (SCAR). Using the developed pair of SCAR primers, the SCAR fragment specific to P. thornei was easily amplified with DNA extracts obtained from different life stages of the nematode. The described SCAR-PCR-based assay has the potential to be optimized for routine practical diagnostic tests. In addition, the use of a species-specific satellite DNA sequence to distinguish P. thornei from other Pratylenchus spp. is discussed.






Similar content being viewed by others
References
Al-Banna, L., Williamsom, V., & Gadner, S. L. (1997). Phylogenetic analysis of nematodes of the genus Pratylenchus using nuclear 26S rDNA. Molecular Phylogenetics and Evolution, 7, 94–102.
Al-Banna, L., Ploeg, A. T., Williamsom, V. M., & Kaloshian, I. (2004). Discrimination of six Pratylenchus species using PCR and species-specific primers. Journal of Nematology, 36, 142–146.
Amiri, S., Subbotin, S. A., & Moens, M. (2002). Identification of the beet cyst nematode Heterodera schachtii by PCR. European Journal of Plant Pathology, 108, 497–506.
Andrés, M. F., Pinochet, J., Hernández-Dorrego, A., & Delibes, A. (2000). Detection and analysis of inter- and intraspecific diversity of Pratylenchus spp. using isozyme markers. Plant Pathology, 49, 640–649.
Barker, K. R. (1998). Introduction and synopsis of advancements in Nematology. In: K. R. Barker, G. A. Pederson, & G. L. Windham (Eds.), Plant nematode interactions (pp. 1–20). Madison, Wisconsin: American Society of Agronomy, Inc.
Barker, K. R., & Noe, J. P. (1987). Establishing and using threshold population levels. In: J. Veech, & D. W. Dickson (Eds.), Vistas on nematology: A commemoration of the twenty-fifth anniversary of the society of nematologists (pp. 75–81). Hyattsville, Maryland: Society of Nematologists, Inc.
Castagnone-Sereno, P., Espárrago, G., Abad, P., Leroy, F., & Bongiovanni, M. (1995). Satellite DNA as a target for PCR-specific detection of the plant parasitic nematode Meloidogyne hapla. Current Genetics, 28, 566–570.
Castagnone-Sereno, P., Leroy, F., & Abad, P. (2000). Cloning and characterization of an extremely conserved satellite DNA family from the root-knot nematode Meloidogyne arenaria. Genome, 43, 346–353.
Castagnone-Sereno, P., Leroy, F., Bongiovanni, M., Zijlstra, C., & Abad, P. (1999). Specific diagnosis of two root-knot nematodes, Meloidogyne chitwoodi and M. fallax, with satellite DNA probes. Phytopathology, 89, 380–384.
Castillo, P., Gómez-Barcina, A., & Jiménez-Díaz, R. M. (1996). Plant parasitic nematodes associated with chickpea in southern Spain and effect of soil temperature on reproduction of Pratylenchus thornei. Nematologica, 42, 211–219.
Castillo, P., Mora-Rodríguez, Mª. P, Navas-Cortés, J. A., & Jiménez-Díaz, R. M. (1998). Interactions between Pratylenchus thornei and Fusarium oxysporum f.sp. ciceris on chickpea. Phytopathology, 88, 836–844.
Castillo, P., Trapero-Casas, J. L., & Jiménez-Díaz, R. M. (1995). Effect of time, temperature, and inoculum density on reproduction of Pratylenchus thornei in carrot disk cultures. Journal of Nematology, 27, 120–124.
Castillo, P., & Vovlas, N. (2002). Factors affecting egg hatch of Heterodera mediterranea and differential responses of olive cultivars to infestation. Journal of Nematology, 34, 146–150.
Da Conceição, I. L. P. M., Dos Santos, M. C. V., Abrantes, I. M. de O., & Santos, M. N. S. (2003). Using RAPD markers to analyse genetic diversity in Portuguese potato cyst nematode populations. Nematology, 5, 137–143.
De Luca, F., Fanelli, E., Di Vito, M., Reyes, A., & De Giorgi, C. (2004). Comparison of the sequences of the D3 expansion of the 26S ribosomal genes reveals different degrees of heterogeneity in different populations and species of Pratylenchus from the Mediterranean region. European Journal of Plant Pathology, 110, 949–957.
Di Vito, M., Greco, N., Halila, H. M., Mabsoute, L., Labdi, M., Beniwal, S. P. S., Saxena, M. C., Singh, K. B., & Solh, M. B. (1994). Nematodes of cool-season food legumes in North Africa. Nematologia Mediterranea, 22, 3–10.
Duncan, L. W., Inserra, R., Thomas, W. K., Dunn, D., Mustika, I., Frisse, L. M., Mendes, M. L., Morris, K., & Kaplan, D. T. (1999). Molecular and morphological analysis of isolates of Pratylenchus coffeae and closely related species. Nematropica, 29, 61–80.
Fourie, H., Zijlstra, C., & McDonald, H. (2001). Identification of root-knot nematode species occurring in South Africa using the SCAR-PCR technique. Nematology, 3(7), 675–680.
Grenier, E., Bossis, M., Fouville, D., Renault, L., & Mugniéry, D. (2001). Molecular approaches to the taxonomic position of Peruvian potato cyst nematodes and gene pool similarities in indigenous and imported populations of Globodera. Heredity, 86, 277–290.
Grenier, E., Laumond, C., & Abad, P. (1995). Characterization of a species-specific satellite DNA from the entomopathogenic nematode Steinernema carpocapsae. Molecular and Biochemical Parasitology, 69, 93–100.
Grenier, E., Castagnone-Sereno, P., & Abad, P. (1997). Satellite DNA sequences as taxonomic markers in nematodes of agronomic interest. Parasitology Today, 13, 398–401.
Hussey, R. S., & Barker, K. R. (1973). A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Disease Reporter, 57, 1025–1028.
Ibrahim, S. K., Perry, R. N., & Webb, R. M. (1995). Use of isozyme and protein phenotypes to discriminate between six Pratylenchus species from Great Britain. Annals of Applied Biology, 126, 317–327.
Jatala, P., & Bridge, J. (1990). Nematode parasites of root and tuber crops. In: M. Luc, R. A. Sikora, & J. Bridge (Eds.), Plant parasitic nematodes in subtropical and tropical agriculture (pp. 137–180). Wallingford, UK: CAB International.
LaMondia, J. A. (2003). Interaction of Pratylenchus penetrans and Rhizoctonia fragariae in strawberry black root rot. Journal of Nematology, 35, 17–22.
Loof, P. A. A. (1991). The Family Pratylenchidae Thorne, 1949. In: W. R Nickle (Ed.), Manual of agricultural nematology (pp. 363–421). New York: Marcel Dekker, Inc.
Mai, W. F., Brodie, B. B., Harrison, M. B., & Jatala, P. (1981). Nematodes. In: W. J. Hooker (Ed.), Compendium of potato diseases (pp. 98–101). St. Paul, Minnesota: The American Phytopathological Society Press.
Nicol, J. M., Davies, K. A., Hancock, T. W., & Fisher, J. M. (1999). Yield loss caused by Pratylenchus thornei on wheat in South Australia. Journal of Nematology, 31, 367–376.
Nicol, J. M., & Ortiz-Monasterio, I. (2004). Effects of the root-lesion nematode, Pratylenchus thornei, on wheat yields in Mexico. Nematology, 6, 485–493.
Ouri, Y., & Mizukubo, T. (1999). Discrimination of seven Pratylenchus species (Nematoda: Pratylenchidae) in Japan by PCR-RFLP analysis. Applied Entomology and Zoology, 34, 205–211.
Paran, I., & Michelmore, R. W. (1993). Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theoretical Applied Genetics, 85, 985–993.
Petersen, D. J., & Vrain, T. C. (1996). Rapid identification of Meloidogyne chitwoodi, M. hapla and M. fallax using PCR primers to amplify their ribosomal intergenic spacer. Fundamental and Applied Nematology, 19, 601–605.
Pinochet, J., Cenis, J. L., Fernández, C., Doucet, M. E., & Marull, M. (1994). Reproductive fitness and Random Amplified Polymorphic DNA variation among isolates of Pratylenchus vulnus. Journal of Nematology, 26, 271–277.
Piotte, C., Castagnone Sereno, P., Bongiovani, M., Dalmasso, A., & Abad, P. (1995). Analysis of a satellite DNA from Meloidogyne hapla and its use as a diagnostic probe. Phytopathology, 85, 458–462.
Potter, J. W., & Olthof, T. H. A. (1993). Nematode pest of vegetable crops. In: K. Evans, D. L. Trudgill, & J. M. Webster (Eds.), Plant Parasitic Nematodes in Temperate Agriculture (pp. 171–207). Wallingford, UK: CAB International.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning, a laboratory manual. New York, USA: Cold Spring Harbor Laboratory Press.
Sharma, S. B., Smith, D. H., & McDonald, D. (1992). Nematode constraints of chickpea and pigeon pea production in the semiarid tropics. Plant Disease, 76, 868–874.
Smiley, R. W., Whittaker, R. G., Gourlie, J. A., & Easley, S. A. (2005). Pratylenchus thornei associated with reduced wheat yield in Oregon. Journal of Nematology, 37, 45–54.
Stack, C. M., Easwaramoorthy, S. G., Metha, U. K., Downes, M. J., Griffin, C. T., & Burnell, A. M. (2000). Molecular characterisation of Heterorhabditis indica isolates from India, Kenya, Indonesia and Cuba. Nematology, 2, 477–487.
Stanton, J., Hugall, A., & Moritz, C. (1997). Nucleotide polymorphisms and an improved PCR-based mtDNA diagnostic for parthenogenetic root-knot nematodes (Meloidogyne spp.). Fundamental and Applied Nematology, 20, 261–268.
Uehara, T., Mizukubo, T., Kushida, A., & Momota, Y. (1998). Identification of Pratylenchus coffeae and P. loosi using specific primers for PCR amplification of ribosomal DNA. Nematologica, 44, 357–368.
Waeyenberge, L., Ryss, A., Moens, M., Pinochet, J., & Vrain, T. C. (2000). Molecular characterisation of 18 Pratylenchus species using rDNA Restriction Fragment Length Polymorphism. Nematology, 2, 135–142.
Williamson, V. M., Caswell-Chen, E. P., Westerdahl, B. B., Wu, F. F., & Caryl, G. (1997). A PCR assay to identify and distinguish single juveniles of Meloidogyne hapla and M. chitwoodi. Journal of Nematology, 29, 9–15.
Zijlstra, C. (1997). A fast PCR assay to identify Meloidogyne hapla, M. chitwoodi and M. fallax, and to sensitively differentiate them from each other and from M. incognita in mixtures. Fundamental and Applied Nematology, 20, 505–511.
Zijlstra, C. (2000). Identification of Meloidogyne chitwoodi, M. fallax and M. hapla based on SCAR-PCR: a powerful way of enabling reliable identification of populations or individuals that share common traits. European Journal of Plant Pathology, 106, 283–290.
Zijlstra, C., Donkers-Venne, D. T. H. M., & Fargette, M. (2000). Identification of Meloidogyne incognita, M, javanica and M. arenaria using sequence characterised amplified region (SCAR) based PCR assays. Nematology, 2(8), 847–853.
Acknowledgements
This research was supported by grant AGF98-0878 from Comisión Interministerial de Ciencia y Tecnología (CICYT) of Spain. We thank Dr J. Mercado Blanco for his comments and suggestions, and A. Valverde Corredor for his technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Carrasco-Ballesteros, S., Castillo, P., Adams, B.J. et al. Identification of Pratylenchus thornei, the cereal and legume root-lesion nematode, based on SCAR-PCR and satellite DNA. Eur J Plant Pathol 118, 115–125 (2007). https://doi.org/10.1007/s10658-007-9110-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10658-007-9110-3