Abstract
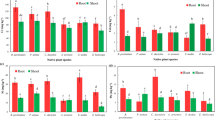
The present investigation is the first in situ comparative study for the identification of Ni and Cu accumulation strategies involved in Odontarrhena obovata (syn. Alyssum obovatum (C.A. Mey.) Turcz.) growing in Cu-rich smelter-influenced (CSI) and non-Cu-influenced (NCI) sites. The total and Na2EDTA (disodium ethylenediaminetetraacetic acid)-extractable metal concentration in soils and plant tissues (roots, stem, leaves and flowers) were determined for CSI and NCI sites. High concentrations of total Ni, Cr, Co and Mg in the soil suggest serpentine nature of both the sites. In spite of high total and extractable Cu concentrations in CSI soil, majority of its accumulation was restricted to O. obovata roots showing its excluder response. Since the translocation and bioconcentration factors of Ni > 1 and the foliar Ni concentration > 1000 μg g−1, it can be assumed that O. obovata has Ni hyperaccumulation potential for both the sites. No significant differences in chlorophyll content in O. obovata leaves were observed between studied sites, suggesting higher tolerance of this species under prolonged heavy metal stress. Furthermore, this species from CSI site demonstrated rather high viability under extreme technogenic conditions due to active formation of antioxidants such as ascorbate, free proline and protein thiols. The presence of Cu in higher concentration in serpentine soil does not exert detrimental effect on O. obovata and its Ni hyperaccumulation ability. Thus, O. obovata could act as a putative plant species for the remediation of Cu-rich/influenced serpentine soils without compromising its Ni content and vitality.


Similar content being viewed by others
Abbreviations
- BCF:
-
Bioconcentration factor
- Chl:
-
Chlorophyll
- CSI:
-
Cu-rich smelter-influenced
- DTNB:
-
5,5-Ditiobis(2-nitrobenzoic acid)
- DW:
-
Dry weight
- EC:
-
Electrical conductivity
- ICP-AES:
-
Inductively coupled plasma atomic emission spectrometer
- MDA:
-
Malondialdehyde
- NCI:
-
Non-Cu-influenced
- Na2EDTA:
-
Disodium ethylenediaminetetraacetic acid
- NPT:
-
Non-protein thiols
- SP:
-
Soluble protein
- SPT:
-
Soluble protein thiols
- TBA:
-
Thiobarbituric acid
- TF:
-
Translocation factor
References
Alloway, B. J. (1990). Heavy metals in soils. New York: Wiley.
Alloway, B. J. (2013). Heavy metals in soils: Trace metals and metalloids in soils and their bioavailability. Environmental pollution (Vol. 22). Dordrecht: Springer.
Baker, D. E., & Senef, J. P. (1995). Copper. In B. J. Alloway (Ed.), Heavy metals in soils (pp. 179–205). London: Blackie Academic and Professional.
Bani, A., Echevarria, G., Sulce, S., Morel, J. L., & Mullai, A. (2007). In situ phytoextraction of Ni by a native population of Alyssum murale on an ultramafic site Albania. Plant and Soil, 293, 79–89.
Bhattacharjee, S. (2005). Reactive oxygen species and oxidative burst: Roles in stress, senescence and signal. Current Science, 89, 1113–1121.
Borisova, G., Chukina, N., Maleva, M., Kumar, A., & Prasad, M. N. V. (2016). Thiols as biomarkers of heavy metal tolerance in the aquatic macrophytes of Middle Urals, Russia. International Journal of Phytoremediation, 18(10), 1037–1045.
Bradford, M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annals of Biochemistry, 72, 248–254.
Broadhurst, C. L., Chaney, R. L., Angle, J. A., Maugel, T. K., Erbe, E. F., & Murphy, C. A. (2004). Simultaneous hyperaccumulation of nickel, manganese and calcium in Alyssum leaf trichomes. Environmental Science and Technology, 38, 5797–5802.
Broadhurst, C. L., Tappero, R. V., Maugel, T. K., Erbe, E. F., Sparks, D. L., & Chaney, R. L. (2009). Interaction of nickel and manganese in accumulation and localization in leaves of the Ni hyperaccumulators Alyssum murale and Alyssum corsicum. Plant and Soil, 314, 35–48.
Chen, Z., Ai, Y., Fang, C., Wang, K., Li, W., Liu, S., et al. (2014). Distribution and phytoavailability of heavy metal chemical fractions in artificial soil on rock cut slopes alongside railways. Journal of Hazardous Materials, 273, 165–173.
Chipeng, K. F., Hermans, C., Colinet, G., Faucon, M. P., Luhembwe, N. M., Meerts, P., et al. (2010). Copper tolerance in the cuprophyte Haumania strumkatangense (S. Moore) P.A. Duvign. and Plancke. Plant and Soil, 328, 235–244.
Demidchik, V. V., Sokolik, A. I., & Yurin, V. M. (2001). Copper surplus toxicity and plant tolerance. Journal Uspekhi Sovremennoi Biologii, 121, 511–525.
Drozdova, I., Alekseeva-Popova, N., Kataeva, M., Bech, J., & Roca, N. (2019). A study of trace elements in plants of the Polar Urals and Chukotka in the search for metallophyte hyperaccumulators. Geochemistry: Exploration, Environment, Analysis, 19(2), 138–145.
Fazlieva, E. R., Kiseleva, I. S., & Zhuikova, T. V. (2012). Antioxidant activity in the leaves of Melilotus albus and Trifolium medium from man-made disturbed habitats in the Middle Urals under the influence of copper. Russian Journal of Plant Physiology, 59(3), 369–375.
Fiske, C. H., & Subbarow, Y. (1925). The colorimetric determination of phosphorus. Journal of Biological Chemistry, 66, 375–400.
Gall, J. E., & Rajakaruna, N. (2013). The physiology, functional genomics, and applied ecology of heavy metal-tolerant Brassicaceae. In M. Lang (Ed.), Brassicaceae: Characterization, functional genomics and health benefits (pp. 121–148). New York: Nova Science Publishers Inc.
Galuszka, A., Migaszewski, Z. M., Dołęgowska, S., Michalik, A., & Duczmal-Czernikiewicz, A. (2015). Geochemical background of potentially toxic trace elements in soils of the historic copper mining area: A case study from Miedzianka Mt., Holy Cross Mountains, south-central Poland. Environmental Earth Science, 74(6), 4589–4605.
Ghasemi, R., Ghaderian, S. M., & Ebrazeh, S. (2015). Nickel stimulates copper uptake by nickel-hyperaccumulator plants in the genus alyssum. Australian Journal of Botany, 63, 56–64.
Gustafson, F. G. (1956). Absorption of 60Co by leaves of young plants and its translocation through the plant. American Journal of Botany, 43, 157–160.
Hajar, A. S. M. (1987). Comparative ecology of Minuartia verna (L.) Hiern. and Thlaspi alpestre L. in the Southern Pennines, with special reference to heavy-metal tolerance. Ph.D. Thesis, University of Sheffield, UK.
Hochstetter, F. (1860). Dun Mountain Copper Mining Company. Nelson Examiner and New Zealand Chronicle, XIX (34)4.
Homer, F. A., Morrison, R. S., Brooks, R. R., Clemens, J., & Reeves, R. D. (1991). Comparative studies of nickel, cobalt, and copper uptake by some nickel hyperaccumulators of the genus Alyssum. Plant and Soil, 138(2), 195–205.
Kabata-Pendias, A., & Mukherjee, A. B. (2007). Trace elements from soil to human. Heidelberg: Springer.
Kabata-Pendias, A., & Pendias, H. (2001). Trace elements in soils and plants. Florida: CRC Press.
Kramer, U. (2010). Metal hyperaccumulation in plants. Annual Review of Plant Physiology and Plant Molecular Biology, 61, 517–534.
Kumar, A., & Maiti, S. K. (2015). Assessment of potentially toxic heavy metal contamination in agricultural fields, sediment, and water from an abandoned chromite-asbestos mine waste of Roro hill, Chaibasa, India. Environmental Earth Science, 74(3), 2617–2633.
Kumar, A., Maiti, S. K., Prasad, M. N. V., & Singh, R. S. (2017). Grasses and legumes facilitate phytoremediation of metalliferous soils in the vicinity of an abandoned chromite–asbestos mine. Journal of Soils and Sediments, 17(5), 1358–1368.
Kumar, A., Tripti, Maleva, M., Kiseleva, I., Maiti, S. K., & Morozova, M. (2019). Toxic metal (loid) s contamination and potential human health risk assessment in the vicinity of century-old copper smelter, Karabash, Russia. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-019-00414-3.
Lange, B., Pourret, O., Meerts, P., Jitaru, P., Cances, B., Grison, C., et al. (2016). Copper and cobalt mobility in soil and accumulation in a metallophyte as influenced by experimental manipulation of soil chemical factors. Chemosphere, 146, 75–84.
Lee, J. (1977). Phytochemical and biogeochemical studies on nickel accumulation by some New Caledonian plants. Ph.D. Thesis, Massey University, Palmerston North, NZ.
Li, Y. M., Chaney, R., Brewer, E., Roseberg, R., Angle, J. S., Baker, A., et al. (2003). Development of a technology for commercial phytoextraction of nickel: Economic and technical considerations. Plant and Soil, 249(1), 107–115.
Lichtenthaler, H. K. (1987). Chlorophylls and carotenoids: Pigments of photosynthetic membranes. Methods in Enzymology, 148, 350–382.
Malaisse, F., Grégoire, J., Brooks, R. R., Morrison, R. S., & Reeves, R. D. (1978). Aeolanthus biformifolius: A hyperaccumulator of copper from Zaire. Science, 199, 887–888.
Maleva, M., Borisova, G., Chukina, N., Kumar, A., & Prasad, M. N. V. (2016). High dose of urea enchances the nickel and copper toxicity in Brazilian elodea (Egeria densa Planch. Casp.). Brazillian Journal of Botany, 39(3), 965–972.
Maleva, M., Garmash, E., Chukina, N., Malec, P., Waloszek, A., & Strzałka, K. (2018). Effect of the exogenous anthocyanin extract on key metabolic pathways and antioxidant status of Brazilian elodea (Egeria densa (Planch.) Casp.) exposed to cadmium and manganese. Ecotoxicology and Environmental Safety, 160, 197–206.
Maleva, M. G., Nekrasova, G. F., Borisova, G. G., Chukina, N. V., & Ushakova, O. S. (2012). Effect of heavy metals on photosynthetic apparatus and antioxidant status of Elodea. Russian Journal of Plant Physiology, 59, 190–197.
Marescotti, P., Carbone, C., De Capitani, L., Grieco, G., Lucchetti, G., & Servida, D. (2008). Mineralogical and geochemical characterisation of open-air tailing and waste-rock dumps from the Libiola Fe-Cu sulphide mine (Eastern Liguria, Italy). Environmental Geology, 53(8), 1613–1626.
Marescotti, P., Comodi, P., Crispini, L., Gigli, L., Zucchini, A., & Fornasaro, S. (2019). Potentially toxic elements in ultramafic soils: A study from metamorphic ophiolites of the Voltri Massif (Western Alps, Italy). Minerals, 9(8), 502.
Marschner, H. (1995). Mineral nutrition of higher plants. London: Academic Press Ltd.
Marsili, S., Roccotiello, E., Carbone, C., Marescotti, P., Cornara, L., & Mariotti, M. G. (2009). Plant colonization on a contaminated serpentine site. Northeastern Naturalist, 16(sp5), 297–308.
Meharg, A. A. (2005). Mechanisms of plant resistance to metal and metalloid ions and potential biotechnological applications. Plant and Soil, 274, 163–174.
Morais, I., Campos, J. S., Favas, P. J. C., Pratas, J., Pita, F., & Prasad, M. N. V. (2015). Nickel accumulation by Alyssum serpyllifolium subsp. lusitanicum (Brassicaceae) from serpentine soils of Bragança and Morais (Portugal) ultramafic massifs: plant–soil relationships and prospects for phytomining. Austrailan Journal of Botany, 63(2), 17–30.
Mukhopadhyay, S., Rana, V., Kumar, A., & Maiti, S. K. (2017). Biodiversity variability and metal accumulation strategies in plants spontaneously inhibiting fly ash lagoon, India. Environmental Science and Pollution Research, 24(29), 22990–23005.
Pilon-Smits, E. (2005). Phytoremediation. Annual Review of Plant Biology, 56, 15–39.
Polley, J. R. (1954). Colorimetric determination of nitrogen in biological materials. Analytical Chemistry, 26(9), 1523–1524.
Pourret, O., Lange, B., Bonhoure, J., Colinet, G., Decree, S., Mahy, G., et al. (2016). Assessment of soil metal distribution and environmental impact of mining in Katanga (Democratic Republic of Congo). Applied Geochemistry, 64, 43–55.
Radojevic, A. A., Serbula, S. M., Kalinovic, T. S., Kalinovic, J. V., Steharnik, M. M., Petrovic, J. V., et al. (2017). Metal/metalloid content in plant parts and soils of Corylus spp. influenced by mining–metallurgical production of copper. Environmental Science and Pollution Research, 24(11), 10326–10340.
Rajakaruna, N., & Bohm, B. A. (2002). Serpentine and its vegetation: A preliminary study from Sri Lanka. Journal of Applied Botany, 76, 20–28.
Reeves, R. D. (2006). Hyperaccumulation of trace elements by plants. In: J. L. Morel, G. Echevarria, N. Goncharova (Eds.), Phytoremediation of metal–contaminated soils (pp. 25–52). Proceedings of the NATO Advanced Study Institute, Třešt’ Castle, Czech Republic, NATO Science Series: IV: Earth and Environmental Sciences 68. Berlin: Springer.
Roccotiello, E., Serrano, H. C., Mariotti, M. G., & Branquinho, C. (2015). Nickel phytoremediation potential of the Mediterranean Alyssoides utriculata (L.) Medik. Chemosphere, 119, 1372–1378.
Roccotiello, E., Zotti, M., Mesiti, S., Marescotti, P., Carbone, C., Cornara, L., et al. (2010). Biodiversity in metal-polluted soils. Fresenius Environmental Bulletin, 19(10b), 2420–2425.
Schat, H., & Vooijs, R. (1997). Multiple tolerance and co-tolerance to heavy metals in Silene vulgaris: a co-segregation analysis. New Phytologist, 136(3), 489–496.
Schutzendubel, A., & Polle, A. (2002). Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. Journal of Experimental Botany, 53(372), 1351–1365.
Shepherd, P. R. (1983). Biogeochemical and geobotanical studies of the ultramafic areas of North Cape, BSc (Hons.) Report, Massey University, Palmerston North, New Zealand.
Stowhas, T., Verdejo, J., Yáñez, C., Celis-Diez, J. L., Martínez, C. E., & Neaman, A. (2018). Zinc alleviates copper toxicity to symbiotic nitrogen fixation in agricultural soil affected by copper mining in central Chile. Chemosphere, 209, 960–963.
Teptina, A. Y., & Paukov, A. G. (2015). Nickel accumulation by species of Alyssum and Noccaea (Brassicaceae) from ultramafic soils in the Urals, Russia. Australian Journal of Botany, 63(2), 78–84.
Udachin, V., Williamson, B. J., Purvis, O. W., Spiro, B., Dubbin, W., Brooks, S., et al. (2003). Assessment of environmental impacts of active smelter operations and abandoned mines in Karabash, Ural Mountains of Russia. Sustainable Development, 11(3), 133–142.
Udachin, V., Yershov, V. & Lansiart, M. (1998). Environmental conditions in the area of exploited massive sulphide deposits of the South Ural. Final report for TACIS Contract FINRUS 9602.
Van der Ent, A., Baker, A. J. M., Reeves, R. D., Pollard, A. J., & Schat, H. (2013). Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant and Soil, 362(1–2), 319–333.
Van der Ent, A., & Reeves, R. D. (2015). Foliar metal accumulation in plants from copper-rich ultramafic outcrops: Case studies from Malaysia and Brazil. Plant and Soil, 389, 401–418.
Von Frenckell-Insam, B. A. K., & Hutchinson, T. (1993). Nickel and zinc tolerance and co-tolerance in Deschampsia cespitosa (L.) Beauv. Subject to artificial selection. New Phytologist, 125, 547–553.
Williamson, B. J., Udachin, V., Purvis, O. W., Spiro, B., Cressey, G., & Jones, G. C. (2004). Characterisation of airborne particulate pollution in the Cu smelter and former mining town of Karabash, South Ural Mountains of Russia. Environmental Monitoring and Assessment, 98(1–3), 235–259.
Acknowledgement
The authors are grateful to Anzhelika Teptina for the identification of plant species and expert reviewers for proofreading the article.
Funding
The work was funded by RFBR and DST according to the research Project No. 19-516-45006. The authors also acknowledge financial support by the Ministry of Science and Higher Education of the Russian Federation (Agreement No. 02.A03.21.0006).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tripti, Kumar, A., Maleva, M. et al. Nickel and copper accumulation strategies in Odontarrhena obovata growing on copper smelter-influenced and non-influenced serpentine soils: a comparative field study. Environ Geochem Health 43, 1401–1413 (2021). https://doi.org/10.1007/s10653-020-00575-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-020-00575-6