Abstract
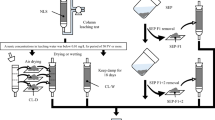
It is important that hazardous excavated sedimentary and metamorphic rocks are treated appropriately and reused without posing an environmental risk. Up-flow column leaching tests were conducted to examine whether arsenic leaching behavior varied among five hazardous excavated sedimentary and metamorphic rocks (two mudstones, clay sediment of marine origin, slate, and black schist) and to determine whether the potential amount of arsenic leaching could be estimated based on the arsenic-bearing mineral phases in the rock. Changes in arsenic concentration with pore volume (PV) showed the same pattern across all rock types, except for one that contained an extremely low amount of water-soluble arsenic, exhibiting an initial increase to reach a peak, followed by a decrease. The arsenic amounts leached before and after the PV at which the arsenic concentration peaked, corresponded to 88% ± 20% of the amount of arsenic fraction 1 obtained by sequential extraction and 76% ± 10% of the amount of arsenic fraction 2, respectively, while the potential amount of arsenic leaching corresponded to 65–89% of the summed total of arsenic fractions 1 + 2. These findings indicate that arsenic exhibits the same leaching behavior among different types of hazardous excavated sedimentary and metamorphic rocks except where extremely low amounts of water-soluble arsenic are present and that the potential amount of arsenic leaching can be approximated by calculating the summed total of arsenic fractions 1 + 2, which allows us to estimate the minimum amount of material required for treatments such as immobilization conducted to prevent arsenic leaching.





Similar content being viewed by others
References
Abdul, K. S. M., Jayasinghe, S. S., Chandana, E. P. S., Jayasumana, C., & Silva, P. M. C. S. D. (2015). Arsenic and human health effects: A review. Environmental Toxicology and Pharmacology,40, 828–846.
Alexakis, D. (2011). Diagnosis of stream sediment quality and assessment of toxic element contamination sources in East Attica, Greece. Environmental Earth Science,63, 1369–1383.
Alexakis, D., & Gamvroula, D. (2014). Arsenic, chromium, and other potentially toxic elements in the rocks and sediments of Oropos-Kalamos basin, Attica, Greece. Applied and Environmental Soil Science,2014, 718534.
Bacon, J. R., & Davidson, C. M. (2008). Is there a future for sequential chemical extraction? The Royal Society of Chemistry,133, 25–46.
Berg, M., Tran, H. C., Nguyen, T. C., Pham, H. V., Schertenleib, R., & Giger, W. (2001). Arsenic contamination of groundwater and drinking water in Vietanam: A human health threat. Environmental Science and Technology,35, 2621–2626.
Cappuyns, V., & Swennen, R. (2008). The use of leaching tests to study the potential mobilization of heavy metals from soils and sediments: A comparison. Water, Air, and Soil pollution,191, 95–111.
Cheng, H., Hu, Y., Luo, J., Xu, B., & Zhao, J. (2009). Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems (Review). Journal of Hazardous Materials,165, 13–26.
Dong, H., Guan, X., Wang, D. W., & Ma, J. (2011). Individual and combined influence of calcium and anions on simultaneous removal of chromate and arsenate by Fe(II) under suboxic conditions. Separation and Purification Technology,80, 284–292.
Drahota, P., Grosslova, Z., & Kindlova, H. (2014). Selectivity assessment of an arsenic sequential extraction procedure for evaluating mobility in mine wastes. Analytica Chimica Acta,839, 34–43.
Elizalde-Gonzalez, M. P., Mattusch, J., Einicke, W. D., & Wennrich, R. (2001). Sorption on soils for arsenic removal. Chemical Engineering Journal,81, 187–195.
Fakhreddine, S., Dittmar, J., Phipps, D., Dadakis, J., & Fendorf, S. (2015). Geochemical triggers of arsenic mobilization during managed aquifer recharge. Environmental Science and Technology,49, 7802–7809.
Gimenez, J., Martinez, M., DePablo, J., Rovira, M., & Duro, L. (2007). Arsenic sorption onto natural hematite, magnetite, and goethite. Journal of Hazardous Materials,141, 575–580.
Guan, X., Ma, J., Dong, H., & Jiang, L. (2009). Removal of arsenic from water: Effect of calcium ions on As(III) removal in the KMnO4-Fe(II) process. Water Research,43, 5119–5128.
Hashem, M. A., Toda, K., & Ohira, S. (2015). Leaching behavior of arsenite and arsenate from the contaminated sediment by the effect of phosphate ion under anaerobic conditions. Environmental Earth Sciences,74, 737–743.
Hossain, M. F. (2006). Arsenic contamination in Bangladesh—An overview. Agriculture, Ecosystems & Environment,113, 1–16.
Hsu, L. I., Cheng, Y. W., Chen, C. J., Wu, M. M., Hsu, K. H., Chiou, H. Y., et al. (2016). Cumulative arsenic exposure is associated with fungal infections: Two cohort studies based on southwestern and northeastern basins in Taiwan. Environment International,96, 173–179.
Igarashi, T., Imagawa, H., Uchiyama, H., & Asakura, K. (2008). Leaching behavior of arsenic from various rocks by controlling geochemical conditions. Minerals Engineering,21, 191–199.
Kamata, A., & Katoh, M. (2019). Arsenic release from marine sedimentary rock after excavation from urbanized coastal areas: Oxidation of framboidal pyrite and subsequent natural suppression of arsenic release. Science of the Total Environment,670, 752–759.
Kanematsu, M., Young, T. M., Fukushi, K., Green, P. G., & Darby, J. L. (2013). Arsenic (III, V)adsorption on a goethite-based adsorbent in the presence of major co-existing ions: Modeling competitive adsorption consistent with spectroscopic and molecular evidence. Geochimica et Cosmochimica Acta,106, 404–428.
Katoh, M., Kitahara, W., & Sato, T. (2014). Sorption of lead in animal manure compost: Contribution of inorganic and organic fractions. Water, Air, and Soil pollution,225, 1828.
Katoh, M., Masaki, S., & Sato, T. (2012). Single-step extraction to determine soluble lead levels in soil. International Journal of GEOMATE,3, 375–380.
Katoh, M., Moriguchi, S., Takagi, N., Akashi, Y., & Sato, T. (2018). Simultaneous control of cadmium release and acidic pH neutralization in excavated sedimentary rock with concurrent oxidation of pyrite using steel slag. Journal of Soils and Sediments,18, 1194–1204.
Katsumi, T. (2015). Soil excavation and reclamation in civil engineering: Environmental aspects. Soil Science and Plant Nutrition,61, 22–29.
Kocar, B. D., Herbel, M. J., Tufano, K. J., & Fendorf, S. (2006). Contrasting effects of dissimilatory iron(II) and arsenic(V) reduction on arsenic retention and transport. Environmental Science and Technology,40, 6715–6721.
Komarek, M., Vanek, A., & Ettler, V. (2013). Chemical stabilization of metals and arsenic in contaminated soils using oxides-A review. Environmental Pollution,172, 9–22.
Leszczynska, D., & Ahnmad, H. (2006). Toxic elements in soil and groundwater: Short-time study on electrokinetic removal of arsenic in the presence of other ions. International Journal of Environmental Research and Public Health,3, 196–201.
Li, J., Kosugi, T., Riya, S., Hshimoto, Y., Hou, H., Terada, A., et al. (2016). Potential for leaching of arsenic from excavated rock after different drying treatments. Chemoshere,154, 276–282.
Li, J., Kosugi, T., Riya, S., Hashimoto, Y., Hou, H., Terada, A., et al. (2017). Use of batch leaching tests to quantify arsenic release from excavated urban soils with relatively low levels of arsenic. Journal of Soils and Sediments,17, 2136–2143.
Li, J., Kosugi, T., Riya, S., Hashimoto, Y., Hou, H., Terada, A., et al. (2018). Pollution potential leaching index as a tool to assess water-leaching risk of arsenic in excavated urban soils. Ecotoxicology and Environmental Safety,147, 72–79.
Lin, S., Yang, H., Na, Z., & Lin, K. (2018). A novel biodegradable arsenic adsorbent by immobilization of iron oxyhydroxide (FeOOH) on the root powder of long-root Eichhornia crassipes. Chemosphere,192, 258–266.
Meng, X., Bang, S., & Korfiatis, G. P. (2000). Effects of silicate, sulfate, and carbonate on arsenic removal by ferric chloride. Water Research,34, 1255–1261.
Mihaljevic, M., Ettler, V., Sisr, L., Sebek, O., Strnad, L., & Vonaskova, V. (2009). Effect of low concentrations of phosphate ions on extraction of arsenic from naturally contaminated soil. Bulletin of Environmental Contamination and Toxicology,83, 422–427.
Nagar, R., Sakar, D., Makris, K. C., & Datta, R. (2010). Effect of solution chemistry on arsenic sorption by Fe- and Al-based drinking-water treatment residuals. Chemosphere,78, 1028–1035.
Ogawa, S., Katoh, M., & Sato, T. (2014). Contribution of hydroxyapatite and ferrihydrite in combined applications for the removal of lead and antimony from aqueous solutions. Water, Air, and Soil pollution,225, 2023.
Ogawa, S., Katoh, M., & Sato, T. (2015). Simultaneous lead and antimony immobilization in shooting range soil by a combined application of hydroxyapatite and ferrihydrite. Environmental Technology,36, 2647–2656.
Parkhurst, D. L., & Appelo, C. A. J. (2013). Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: U.S. Geological Survey Techniques and Methods 6, 43. Available at: https://pubs.usgs.gov/tm/06/a43/.
Rahman, M. A., & Hasegawa, H. (2011). High levels of inorganic arsenic in rice in areas where arsenic-contaminated water is used for irrigation and cooking. Science of the Total Environment,409, 4645–4655.
Razo, I., Carrizales, L., Castro, J., Fernando, D. B., & Monroy, M. (2004). Arsenic and heavy metal pollution of soil, water and sediments in a semiarid climate mining area in Mexico. Water, Air, and Soil pollution,152, 129–152.
Sharma, P., & Kappler, A. (2011). Desorption of arsenic from clay and humic acid-coated clay by dissolved phosphate and silicate. Journal of Contaminant Hydrology,126, 216–225.
Shuman, L. M. (1985). Fractionation method for soil microelements. Soil Science,140, 11–22.
Tabelin, C. B., Basri, A. H. M., Igarashi, T., & Yoneda, T. (2012a). Removal of arsenic, boron, and selenium from excavated rocks by consecutive washing. Water, Air, and Soil pollution,223, 4153–4167.
Tabelin, C. B., Hashimoto, A., Igarashi, T., & Yoneda, T. (2014a). Leaching of boron, arsenic and selenium from sedimentary rock: I. Effect of contact time, mixing speed and liquid-to-solid ratio. Science of the Total Environment,472, 620–629.
Tabelin, C. B., Hashimoto, A., Igarashi, T., & Yoneda, T. (2014b). Leaching of boron, arsenic and selenium from sedimentary rocks: II. pH dependence, speciation and mechanisms of release. Science of the Total Environment,473–474, 244–253.
Tabelin, C. B., & Igarashi, T. (2009). Mechanisms of arsenic and lead release from hydrothermally altered rock. Journal of Hazardous Materials,169, 980–990.
Tabelin, C. B., Igarashi, T., & Takahashi, R. (2012b). Mobilization and speciation of arsenic from hydrothermally altered rock in laboratory column experiments under ambient conditions. Applied Geochemistry,27, 326–342.
Tabelin, C. B., Igarashi, T., Villacorte-Tabelin, M., Park, I., Opiso, E. M., Ito, M., et al. (2018). Arsenic, selenium, boron, lead, cadmium, copper, and zinc in naturally contaminated rocks: A review of their sources, modes of enrichment, mechanisms of release, and mitigation strategies. Science of the Total Environment,645, 1522–1553.
Tamoto, S., Tabelin, C. B., Igarashi, T., Ito, M., & Hiroyoshi, N. (2015). Short and long term release mechanisms of arsenic, selenium and boron from a tunnel-excavated sedimentary rock under in situ conditions. Journal of Contaminant Hydrology,175–176, 60–71.
Tangviroon, P., Hayashi, R., & Igarashi, T. (2017). Effects of additional layer(s) on the mobility of arsenic from hydrothermally altered rock in laboratory column experiments. Water, Air, and Soil pollution,228, 191.
Tatsuhara, T., Arima, T., Igarashi, T., & Tabelin, C. B. (2012). Combined neutralization-adsorption system for the disposal of hydrothermally altered excavated rock producing acidic leachate with hazardous elements. Engineering Geology,139–140, 76–84.
Tessier, A., Campbell, P. G. C., & Bisson, M. (1979). Sequential extraction procedure for the speciation of particulate trace metals. Analytical Chemistry,51, 844–851.
Wan, X., Dong, H., Feng, L., Lin, Z., & Luo, Q. (2017). Comparison of three sequential extraction procedures for arsenic fractionation in highly polluted sites. Chemosphere,178, 402–410.
Wen, F., Hou, H., Yao, N., Yan, Z., Bai, L., & Li, F. (2013). Effects of simulated acid, EDTA, or their combination, on migration and chemical fraction distribution of extraneous metals in Ferrosol. Chemosphere,90, 349–357.
Wenzel, W. W., Kirchbaumer, N., Prohaska, T., Stingeder, G., Lombi, E., & Adriano, D. C. (2001). Arsenic fractionation in soils using an improved sequential extraction procedure. Analytica Chimica Acta,436, 309–323.
Wu, Y., Li, W., & Sparks, D. L. (2015). Effect of iron(II) on arsenic sequestration by δ-MnO2: Desorption studies using stirred-flow experiments and X-ray absorption fine-structure spectroscopy. Environmental Science and Technology,49, 13360–13368.
Xu, W., Wang, H., Liu, R., Zhao, X., & Qu, J. (2011). Arsenic release from arsenic-bearing Fe–Mn binary oxide: Effects of Eh condition. Chemosphere,83, 1020–1027.
Yang, G., Liu, Y., & Song, S. (2015). Competitive adsorption of As(V) with co-existing ions on porous hematite in aqueous solutions. Journal of Environmental Chemical Engineering,3, 1497–1503.
Zhang, G., Liu, H., Qu, J., & Jefferson, W. (2012). Arsenate uptake and arsenite simultaneous sorption and oxidation by Fe–Mn binary oxides: Influence of Mn/Fe ratio, pH, Ca2+, and humic acid. Journal of Colloid and Interface Science,366, 141–146.
Acknowledgements
The authors are grateful to Mrs. T. Miura and T. Higasayama for rock sample collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suzuki, S., Katoh, M. Estimation of potential arsenic leaching from its phases in excavated sedimentary and metamorphic rocks. Environ Geochem Health 42, 407–418 (2020). https://doi.org/10.1007/s10653-019-00371-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-019-00371-x