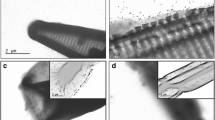
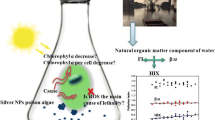
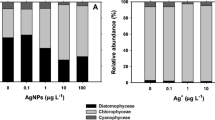
Abstract
The fate and toxicity of silver nanoparticles (AgNPs) and ions in water bodies is largely determined by the natural organic matter (NOM)-mediated redox cycling. However, the process of NOM-mediated redox cycling in the day/night cycles remains elusive. In this study, the inter-transformation between AgNPs and Ag+ ion caused by humic acid (HA) was investigated under controlled light and dark conditions. It was shown that HA induced the reduction of Ag+ into AgNPs in simulated sunlight, and also oxidize AgNPs to release Ag+ in darkness. Kinetics data demonstrated that the rates of AgNPs formation and dissolution increased along with the increment of HA concentrations. Along with the pH increase, the reduction of Ag+ accelerated, but the oxidative dissolution of AgNPs was inhibited. In day-night cycles, the AgNPs and Ag+ concentrations exhibited similar wave-shaped change curves. The peaks of AgNPs and Ag+ ion appeared at 7 p.m. and 7 a.m., respectively. The toxicity of AgNPs/Ag+ to Escherichia coli was determined primarily by the concentration of dissolved Ag+, but also affected by the particle-specific toxicity. The dual role of HA implied that previous reports about the photo-reduction of Ag+ to AgNPs by NOM should be reconsidered, and the oxidizability of HA in darkness strongly affect the transformation and toxicity of AgNPs in water.







Similar content being viewed by others
Change history
13 April 2021
A Correction to this paper has been published: https://doi.org/10.1007/s10646-021-02412-7
References
Adegboyega NF, Sharma VK, Siskova KM, Vecerova R, Kolar M, Zboril R, Gardea Torresdey JL (2014) Enhanced formation of silver nanoparticles in Agt-NOM-Iron(II, III) systems and antibacterial activity studies. Environ Sci Technol 48:3228–3235
Aiken GR, Hsu Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45(8):3196–3201
Blaser SA, Scheringer M, MacLeod M, Hungerbuhler K (2008) Estimation of cumulative aquatic exposure and risk due to silver: contribution of nano-functionalized plastics and textiles. Sci Total Environ 390(2-3):396–409
Benn TM, Westerhoff P (2008) Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol 42:4133–4139
Borm P, Klaessig FC, Landry TD, Moudgil B, Pauluhn J, Thomas K, Trottier R, Wood S (2006) Research strategies for safety evaluation of nanomaterials, part V: role of dissolution in biological fate and effects of nanoscale particles. Toxicol Sci 90(1):23–32
Chao JB, Liu JF, Yu SJ, Feng YD, Tan ZQ, Liu R, Yin YG (2011) Speciation analysis of silver nanoparticles and silver ions in antibacterial products and environmental waters via cloud point extraction-based separation. Anal Chem 83(17):6875–6882
Chinnapongse SL, MacCuspie RI, Hackley VA (2011) Persistence of singly dispersed silver nanoparticles in natural freshwaters, synthetic seawater, and simulated estuarine waters. Sci Total Environ 409:2443–2450
Daniel AL, Abdolahpur MF, Vijver MG, Peijnenburg WJGM (2019) Dissolution and aggregation kinetics of zero valent copper nanoparticles in (simulated) natural surface waters: simultaneous effects of pH, NOM and ionic strength. Chemosphere 226:841–850
Fujii M, Rose AL, Waite TD, Omura T (2010) Oxygen and superoxide-mediated redox kinetics of iron complexed by humic substances in coastal seawater. Environ Sci Technol 44(24):9337–42
Gaberell M, Chin YP, Hug SJ, Sulzberger B (2003) Role of dissolved organic matter composition on the photoreduction of Cr(VI) to Cr(III) in the presence of iron. Environ Sci Technol 37(19):4403–4409
Gu BH, Bian YR, Miller CL, Dong WM, Jiang X, Liang LY (2011) Mercury reduction and complexation by natural organic matter in anoxic environments. Proc Natl Acad Sci USA 108(4):1479–1483
Gunsolus IL, Mousavi MPS, Hussein K, Bühlmann P, Haynes CL (2015) Effects of humic and fulvic acids on silver nanoparticle stability, dissolution, and toxicity. Environ Sci Technol 49:8078–8086
He D, Jones AM, Garg S, Pham AN, Waite TD (2011) Silver nanoparticle−reactive oxygen species interactions: Application of a charging−discharging model. J Phys Chem C 115:5461–5468
Hiriart-Baer VP, Fortin C, Lee DY, Campbell PGC (2006) Toxicity of silver to two freshwater algae, Chlamydomonas reinhardtii and Pseudokirchneriella subcapitata, grown under continuous culture conditions: Influence of thiosulphate. Aquat Toxicol 78:136–148
Hwang ET, Lee JH, Chae YJ, Kim YS, Kim BC, Sang BI, Gu MB (2008) Analysis of the toxic mode of action of silver nanoparticles using stress-specific bioluminescent bacteria. Small 4:746–750
Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, Siegrist H (2011) Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ Sci Technol 45:3902–3908
Levard C, Mitra S, Yang T, Jew AD, Badireddy AR, Lowry GV, Brown GE (2013) Effect of chloride on the dissolution rate of silver nanoparticles and toxicity to E. coli. Environ Sci Technol 47:5738–5745
Li L, Hartmann G, Do blinger M, Schuster M (2013) Quantification of nanoscale silver particles removal and release from municipal wastewater treatment plants in Germany. Environ Sci Technol 47:7317–7323
Li Y, Niu J, Shang E, Crittenden J (2014) Photochemical transformation and photoinduced toxicity reduction of silver nanoparticles in the presence of perfluorocarboxylic acids under UV irradiation. Environ Sci Technol 48:4946–4953
Li X, Schirmer K, Bernard L, Sigg L, Pillai S, Behra R (2015) Silver nanoparticle toxicity and association with the alga Euglena gracilis. Environ Sci Nano 2:594–602
Liu H, Gu X, Wei C, Fu H, Alvarez PJJ, Li Q, Zheng S, Qu X, Zhu D (2018) Threshold concentrations of silver ions exist for the sunlight-induced formation of silver nanoparticles in the presence of natural organic matter. Environ Sci Technol 52:4040–4050
Liu J, Hurt RH (2010) Ion release kinetics and particle persistencein aqueous nano-silver colloids. Environ Sci Technol 44(6):2169–2175
Marambio-Jones C, Hoek EMV (2010) A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J Nanopart Res 12:1531–1551
Misra SK, Dybowska A, Berhanu D, Luoma SN, Valsami Jones E (2012) The complexity of nanoparticle dissolution and its importance in nanotoxicological studies. Sci Total Environ 438:225–232
Molleman B, Hiemstra T (2017) Time, pH, and size dependency of silver nanoparticle dissolution: the road to equilibrium. Environ Sci Nano 4:1314–1327
Navarro E, Piccapietra F, Wagner B, Marconi F, Kaegi R, Odzak N, Sigg L, Behra R (2008) Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ Sci Technol 42(23):8959–8964
Ng CK, Sivakumar K, Liu X, Madhaiyan M, Ji LH, Yang L, Tang CY, Song H, Kjelleberg S, Cao B (2013) Influence of outer membrane c-type cytochromes on particle size and activity of extracellular nanoparticles produced by Shewanella oneidensis. Biotechnol Bioeng 110:1831–1837
Palenova EE, Belogub EV, Plotinskaya OY, Novoselov KA, Maslennikov VV, Kotlyarov VA, Blinov IA, Kuzmenko AA, Griboedova IG (2015) Chemical evolution of pyrite at the kopylovsky and kavkaz black shale-hosted gold deposits, Bodaybo district, Russia: Evidence from EPMA and LA-ICP-MA data. Geol Ore Deposit 57(1):64–84
Pokhrel LR, Dubey B, Scheuerman PR (2013) Impacts of select organic ligands on the colloidal stability, dissolution dynamics, and toxicity of silver nanoparticles. Environ Sci Technol 47:12877–12885
Pokhrel LR, Dubey B, Scheuerman PR (2014) Natural water chemistry (dissolved organic carbon, pH, and hardness) modulates colloidal stability, dissolution, and antimicrobial activity of citrate functionalized silver nanoparticles. Environ Sci Nano 1:45–54
Ratte HT (1999) Bioaccumulation and toxicity of silver compounds: a review. Environ Toxicol Chem 18:89–108
Rose AL, Waite TD (2005) Reduction of organically complexed ferric iron by superoxide in a simulated natural water. Environ Sci Technol 39(8):2645–2650
Rose AL, Waite TD (2006) Role of superoxide in the photochemical reduction of iron in seawater. Geochim Cosmochim Acta 70(15):3869–3882
Shi JP, Xu B, Sun X, Ma CY, Yu CP, Zhang HW (2013) Light induced toxicity reduction of silver nanoparticles to Tetrahymena Pyriformis: Effect of particle size. Aquat Toxicol 132:53–60
Sotiriou GA, Meyer A, Knijnenburg JTN, Panke S, Pratsinis SE (2012) Quantifying the origin of released Ag+ from nanosilver. Langmuir 28(45):15929–15936
Levard C, Hotze EM, Lowry GV, Brown GE (2012) Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ Sci Technol 46(13):6900–6914
Stankus DP, Lohse SE, Hutchison JE, Nason JA (2011) Interactions between natural organic matter and gold nanoparticles stabilized with different organic capping agents. Environ Sci Technol 45(8):3238–3244
Stensberg MC, Madangopal R, Yale G, Wei Q, Ochoa Acuna H, Wei A et al. (2014) Silver nanoparticle-specific mitotoxicity in Daphnia magna. Nanotoxicology 8(8):833–842
Voelker BM, Morel FMM, Sulzberger B (1997) Iron redox cycling in surface waters: effects of humic substances and light. Environ Sci Technol 31(4):1004–1011
Wang Z, Liang L, Gu B (2012) Mercury reduction and oxidation by reduced natural organic matter in anoxic environments. Environ Sci Technol 46:292–299
Xiu ZM, Ma J, Alvarez PJJ (2011) Differential effect of common ligands and molecular oxygen on antimicrobial activity of silver nanoparticles versus silver Ions. Environ Sci Technol 45:9003–9008
Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJJ (2012) Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett 12(8):4271–4275
Yin L, Cheng Y, Espinasse B, Colman BP, Auffan M, Wiesner M, Rose J, Liu J, Bernhardt ES (2011) More than the ions: the effects of silver nanoparticles on Lolium multiflorum. Environ Sci Technol 45:2360–2367
Yu SJ, Yin YG, Chao JB, Shen MH, Liu JF (2013) Highly dynamic PVP-coated silver nanoparticles in aquatic environments: chemical and morphology change induced by oxidation of Ag0 and reduction of Ag+. Environ Sci Technol 1:403–411
Zhang H, Smith JA, Oyanedel Craver V (2012) The effect of natural water conditions on the anti-bacterial performance and stability of silver nanopraticles capped with different polymers. Water Res 46:691–699
Zhou W, Liu YL, Stallworth AM, Ye C, Lenhart JJ (2016) Effects of pH, electrolyte, humic acid, and light exposure on the longterm fate of silver nanoparticles. Environ Sci Technol 50:12214–12224
Acknowledgements
The Science and Technology Planning Project of Hunan Province (Grant No. 2019RS2036); the Changsha Plan Project of Science and Technology (Grant No. kq1801025); and the Hunan Engineering Technology Research Centre for Irrigation Water Purification.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Liu, Y., Li, C., Luo, S. et al. Inter-transformation between silver nanoparticles and Ag+ induced by humic acid under light or dark conditions. Ecotoxicology 30, 1376–1385 (2021). https://doi.org/10.1007/s10646-020-02284-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-020-02284-3