Abstract
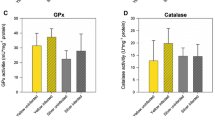
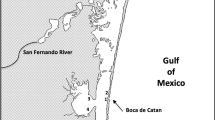
The aims of the present study were to compare the health status of yellow eels (Anguilla anguilla) developing in three estuaries of the NW Portuguese coast with different levels of pollution and their physiological responses to combined effects of environmental variation and pollution. For this, a field study was performed using a multi-parameter approach, including eels condition indexes and biomarkers, water quality variables and other environmental factors. Sixteen biological parameters were assessed, namely: hepatosomatic index (LSI), Fulton’s condition index (K), lipid peroxidation (LPO), total glutathione (TG), reduced glutathione (GSH), oxidised glutathione (GSSG), GSH/GSSG, and the activity of the enzymes acetylcholinesterase (AChE), lactate dehydrogenase (LDH), sodium-potassium ATPase (Na+/K+-ATPase), ethoxyresorufin O-deethylase (EROD), glutathione S-transferases (GST), catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx) and glutathione reductase (GR). Ten environmental factors were also measured in water: temperature, salinity, pH, phosphates, nitrates, nitrites, ammonium, silica, phenol and hardness. Globally, the biomarkers indicate exposure and toxic effects of pollutants on eels living in contaminated estuaries. The relationships between biological and environmental variables were assessed through redundancy analysis. K and LSI indexes, AChE and Na+/K+-ATPase, total glutathione levels and the antioxidant enzymes CAT, GR, and SOD where the factors most discriminating reference (Minho River estuary) from contaminated estuaries (Lima and Douro Rivers estuaries). Moreover, the most striking outcomes of pollutants exposure on biological responses were observed during winter, probably due to a joint effect of cold weather and pollution stress. Altogether, the results indicate that the development of eels in the polluted estuaries of Lima and Douro rivers is interfering with physiological functions determinant for their survival and performance. This may increase the mortality rates during the continental life-phase of the species and decrease the percentage of animals able to successfully complete their oceanic migration and, thus, reduce the contribution of each generation to the next one.





Similar content being viewed by others
References
Almeida CM, Serôdio P, Florêncio MH et al (2007) New strategies to screen for endocrine-disrupting chemicals in the Portuguese marine environment utilizing large volume injectioncapillary gas chromatography–mass spectrometry combined with retention time locking libraries (LVI-GC-MS-RTL). Anal Bioanal Chem 387:2569–2583
Baeta F, Pinheiro A, Corte-Real M et al (2005) Are the fisheries in the Tagus estuary sustainable? Fish Res 76:243–251
Baker MA, Cerniglia GJ, Zaman A (1990) Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190:360–365
Bird RP, Draper AH (1984) Comparative studies on different methods of malondihaldehyde determination. Methods Enzymol 90:105–110
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buet A, Banas D, Vollaire Y et al (2006) Biomarker responses in European eel (Anguilla anguilla) exposed to persistent organic pollutants. A field study in the Vaccares lagoon (Camargue, France). Chemosphere 65:1846–1858
Burke MD, Mayer RT (1974) Ethoxyresorufin: direct fluorimetric assay of a microsomal-o-deethylation which is preferentially inducible by 3-methylcholantrene. Drug Metab Dispos 2:583–588
Cairrão E, Couderchet M, Soares AMVM et al (2004) Glutathione-S-transferase activity of Fucus spp. as a biomarker of environmental contamination. Aquat Toxicol 70:277–286
Cajaraville MP, Bebianno MJ, Blasco J et al (2000) The use of biomarkers to assess the impact of pollution in coastal environments of the Iberian Peninsula: a practical approach. Sci Total Environ 247:295–311
Castro BB, Sobral O, Guilhermino L et al (2004a) An in situ bioassay integrating individual and biochemical responses using small fish species. Ecotoxicology 13:667–681
Castro M, Santos MM, Monteiro NM et al (2004b) Measuring lysosomal stability as an effective tool for marine coastal environmental monitoring. Mar Environ Res 58:741–745
Christie NT, Costa M (1984) In vitro assessment of the toxicity of metal compounds. IV. Disposition of metals in cells: interactions with membranes, glutathione, metallothioneins and DNA. Biol Trace Elem Res 6:139–158
CITES (2008) Convention on international trade in endangered species of wild fauna and flora: apendices I, II, and III. United Nations Environmental Programme, International Environment House, Geneva
Clairborne A (1985) Catalase activity. In: Greenwald RA (ed) CRC handbook of methods in oxygen radical research. CRC Press, Boca Raton, pp 283–284
Cohen A, Nugegoda D, Gagnon MM (2001) Metabolic responses of fish following exposure to two different oil spill remediation techniques. Ecotoxicol Environ Saf 48:306–310
Colombo G, Grandi G (1996) Histological study of the development and sex differentiation of the gonad in the European eel. J Fish Biol 48:493–512
Cossu C, Doyotte A, Babut M et al (2000) Antioxidant biomarkers in freshwater bivalves, Unio tumidus, in response to different contamination profiles of aquatic sediments. Ecotoxicol Environ Saf 5:106–121
Cribb AE, Leeder JS, Spielberg SP (1989) Use of a microplate reader in an assay of glutathione reductase using 5, 5′-dithiobis(2-nitrobenzoic acid). Anal Biochem 183:195–196
Cunha I, Garcia LM, Guilhermino L (2005) Sea-urchin (Paracentrotus lividus) glutathione S-transferases and cholinesterase activities as biomarkers of environmental contamination. J Environ Monit 7:288–294
De Coen WM, Janssen CR, Segner H (2001) The use of biomarkers in Daphnia magna toxicity testing V. In vivo alterations in the carbohydrate metabolism of Daphnia magna exposed to sublethal concentrations of mercury and lindane. Ecotoxicol Environ Saf 48:223–234
Dekker W (2004) Slipping through our hands—population dynamics of the European eel. University of Amsterdam, Amsterdam, p 186
Diamantino TC, Almeida E, Soares AMVM et al (2001) Lactate dehydrogenase activity as an effect criterion in toxicity tests with Daphnia magna straus. Chemosphere 45:553–560
Durif C, Dufour S, Elie P (2005) The silvering process of Anguilla anguilla: a new classification from the yellow resident to the silver migrating stage. J Fish Biol 66:1025–1043
EIFAC/ICES (2007) Report of the 2007 session of the joint EIFAC/ICES working group on eels. In: EIFAC Occasional Paper No 38/ICES CM 2007/ACFM:23. European Inland Fisheries Advisory Commission/International Council for the Exploration of the Sea, Bordeaux/Copenhagen, p 526
Ellman GL, Courtney KD, Andres V Jr et al (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
FAO (2007) Report of the second FAO ad hoc expert advisory panel for the assessment of proposals to amend appendices I and II of CITES concerning commercially-exploited aquatic species. In: FAO Fisheries Report No 833, FAO, Rome, p 133
Ferreira M, Antunes P, Gil O et al (2004) Organochlorine contaminants in flounder (Platichthys flesus) and mullet (Mugil cephalus) from Douro estuary, and their use as sentinel species for environmental monitoring. Aquat Toxicol 69:347–357
Ferreira M, Moradas-Ferreira P, Reis-Henriques MA (2006) The effect of long-term depuration on phase I and phase II biotransformation in mullets (Mugil cephalus) chronically exposed to pollutants in River Douro Estuary, Portugal. Mar Environ Res 61:326–338
Feunteun EE (2002) Management and restoration of European eel population (Anguilla anguilla): an impossible bargain. Ecol Eng 18:575–591
Filho DW, Tribess T, Gáspari C et al (2001) Seasonal changes in antioxidant defenses of the digestive gland of the brown mussel (Perna perna). Aquaculture 203:149–158
Flohé L, Ötting F (1984) Superoxide dismutase assays. Methods Enzymol 105:93–104
Frasco MF, Guilhermino L (2002) Effects of dimethoate and beta-naphthoflavone on selected biomarkers of Poecilia reticulata. Fish Physiol Biochem 26:149–156
Gravato C, Santos MA (2003) Genotoxicity biomarkers’ association with B(a)P biotransformation in Dicentrarchus labrax L. Ecotoxicol Environ Saf 55:352–358
Gravato C, Oliveira M, Santos MA (2005) Oxidative stress and genotoxic responses to resin acids in Mediterranean mussels. Ecotoxicol Environ Saf 61:221–229
Gravato C, Teles M, Oliveira M et al (2006) Oxidative stress, liver biotransformation and genotoxic effects induced by copper in Anguilla anguilla L.—the influence of pre-exposure to beta-naphthoflavone. Chemosphere 65:1821–1830
Gravato C, Faria M, Alves A et al (2007) Biomonitoring studies performed with European eel populations from the estuaries of Minho, Lima and Douro Rivers (NW Portugal). In: Kim YJ, Platt U (eds) Advanced environmental monitoring. Springer, New York, pp 390–401
Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinyl-pyridine. Anal Biochem 106:207–212
Guilhermino L, Lopes MC, Carvalho AP et al (1996) Acetylcholinesterase activity in juveniles of Daphnia magna Straus. Bull Environ Contam Toxicol 57:979–985
Guilhermino L, Barros P, Silva MC et al (1998) Should the use of inhibition of cholinesterase as a specific biomarker for organophosphate and carbamate pesticides be questioned? Biomarkers 3:157–163
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249:7130–7139
HELCOM (2007) HELCOM Red list of threatened and declining species of lampreys and fish of the Baltic Sea. In: Baltic Sea environmental proceedings, p 40
Hoque MT, Yusoff FM, Law AT et al (1998) Effect of hydrogen sulphide on liver somatic index and Fulton’s condition factor in Mystus nemerus. J Fish Biol 52:23–30
ICN (2008) Livro vermelho dos vertebrados de Portugal—Peixes dulciaquícolas e migradores, anfíbios, répteis, aves e mamíferos, 3rd edn. Assírio & Alvim, Lisboa
INAG (2000) Planos das bacias Hidrográficas dos rios Luso-Espanhois—Síntese. Caracterização e Diagnóstico. Instituto da Água, Direcção de Serviços de Recursos Hídricos, Divisão de Recursos Subterrâneos, p 398
INE (2008) Estatísticas da Pesca 2005 e 2006. INE—Instituto Nacional de Estatística, I.P. Dados não publicados, Lisboa
Kennish MJ (2002) Environmental threats and environmental future of estuaries. Environ Conserv 29:78–107
Knights B (1997) Risk assessment and management of contamination of eels (Anguilla spp.) by persistent xenobiotic organochlorine compounds. Chem Ecol 13:171–212
Lemly AD (1993) Metabolic stress during winter increases the toxicity of selenium to fish. Aquat Toxicol 27:133–158
Lemly AD (1996) Winter stress syndrome: an important consideration for hazard assessment of aquatic pollutants. Ecotoxicol Environ Saf 34:223–227
Lepš J, Šmilauer P (2003) Multivariate analysis of ecological data using CANOCO. Cambridge University Press, New York
Lima I, Moreira SM, Osten JR et al (2007) Biochemical responses of the marine mussel Mytilus galloprovincialis to petrochemical environmental contamination along the North-western coast of Portugal. Chemosphere 66:1230–1242
Lionetto MG, Giordano ME, Vilella S et al (2000) Inhibition of eel enzymatic activities by cadmium. Aquat Toxicol 48:561–571
Livingstone DR, Lemaire P, Matthews A et al (1993) Pro-oxidant, antioxidant and 7-ethoxyresofurin O-deethylase (EROD) activity responses in liver of Dab (Limanda limanda) exposed to sediment contaminated with hydrocarbons and other chemicals. Mar Pollut Bull 26:602–606
Lloret J, Gil de Sola L, Souplet A et al (2002) Effects of large-scale habitat variability on condition of demersal exploited fish in the north-western Mediterranean. ICES J Mar Sci 59:1215–1227
Ludke J, Hill E, Dieter M (1975) Cholinesterase (ChE) response and related mortality among birds fed ChE inhibitors. Arch Environ Contam Toxicol 3:1–21
McCormick SD (1993) Methods for non-lethal gill biopsy and measurement of Na+, K+-ATPase activity. Can J Fish Aquat Sci 50:656–658
Mohandas J, Marshall JJ, Duggins GG et al (1984) Differential distribution of glutathione and glutathione related enzymes in rabbit kidney: possible implications in analgesic neuropathy. Cancer Res 44:5086–5091
Monteiro M, Quintaneiro C, Nogueira AJ et al (2007) Impact of chemical exposure on the fish Pomatoschistus microps Kroyer (1838) in estuaries of the Portuguese Northwest coast. Chemosphere 66:514–522
Moreira SM, Moreira-Santos M, Ribeiro R et al (2004) The ‘Coral Bulker’ fuel oil spill on the North coast of Portugal: spatial and temporal biomarker responses in Mytilus galloprovincialis. Ecotoxicology 13:619–630
Moriarty C, Dekker W (1997) Management of the European eel. Fish Bull 15:1–110
Mucha AP, Bordalo AA, Vasconcelos MT (2004) Sediment quality in the Douro river estuary based on trace metal contents, macrobenthic community and elutriate sediment toxicity test (ESTT). J Environ Monit 6:585–592
Ohkawa H (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Oliver JD, Holeton GF, Chua KE (1979) Overwinter mortality of fingerling smallmouth bass in relation to size, relative energy stores, and environmental temperature. Trans Am Fish Soc 108:130–136
Palstra AP, van Ginneken VJT, Murk AJ et al (2006) Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 93:145–148
Payne JF, Mathieu A, Melvin W et al (1996) Acetylcholinesterase, an old biomarker with a newfuture? Field trials in association with two urban rivers and a paper mill in Newfoundland. Mar Pollut Bull 32:225–231
Peña-Llopis S, Pena JB, Sancho E et al (2001) Glutathione-dependent resistence of the European eel Anguilla anguilla to the herbicide molinate. Chemosphere 45:671–681
Pierron F, Baudrimont M, Bossy A et al (2007) Impairment of lipid storage by cadmium in the European eel (Anguilla anguilla). Aquat Toxicol 81:304–311
Quintaneiro C, Monteiro M, Pastorinho R et al (2006) Environmental pollution and natural populations: a biomarkers case study from the Iberian Atlantic coast. Mar Pollut Bull 52:1406–1413
Regoli R, Principato G (1995) Glutathione, glutathione-dependent and antioxidant enzymes in mussel, Mytilus galloprovincialis, exposed to metals under field and laboratory conditions: implications for the use of biochemical biomarkers. Aquat Toxicol 31:143–164
Ribeiro CAO, Vollaire Y, Sanchez-Chardi A et al (2005) Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue nature reserve, France. Aquat Toxicol 74:53–69
Ribeiro C, Tiritan M, Rocha E et al (2008) Seasonal and spatial distribution of several endocrine-disrupting compounds in the Douro river estuary, Portugal. In: Arch Environ Contam Toxicol
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Can 191:1–382
Robinet TT, Feunteun EE (2002) Sublethal effects of exposure to chemical compounds: a cause for the decline in Atlantic eels? Ecotoxicology 11:265–277
Roche H, Buet A, Jonot O et al (2000) Organochlorine residues in european eel (Anguilla anguilla), crucian carp (Carassius carassius) and catfish (Ictalurus nebulosus) from Vaccares lagoon (French National Nature Reserve of Camargue)—effects on some physiological parameters. Aquat Toxicol 48:443–459
Rodrigues P, Reis-Henriques MA, Campos J et al (2006) Urogenital papilla feminization in male Pomatoschistus minutus from two estuaries in northwestern Iberian Peninsula. Mar Environ Res 62(Suppl):S258–S262
Sheppard K, Simkiss K (1978) The effects of heavy metal ions on Ca2+ ATPase extracted from fish gills. Comp Biochem Physiol B 61:69–72
Sherwood L, Klandorf H, Yancey PH (2005) Animal physiology: from genes to organisms, 1st edn. Thomson Brooks/Cole, Belmont
Simon J (2007) Age, growth, and condition of European eel (Anguilla anguilla) from six lakes in the River Havel system (Germany). ICES J Mar Sci 64:1414–1422
Sloof W (1982) Skeletal anomalies in fish from polluted surface waters. Aquat Toxicol 2:157–173
Slooff W, van Kreijl CF, Baars AJ (1983) Relative liver weights and xenobiotic-metabolizing enzymes of fish from polluted surface waters in the Netherlands. Aquat Toxicol 4:10–14
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research, 3rd edn. W. H. Freeman and Company, New York
Stagg RM, Goksoyr A, Rodger G (1992a) Changes in branchial Na+, K+-ATPase, metallothionein and P450 1A1 in dab Limanda limanda in the Germain Bight: indicators of sediment contamination? Mar Ecol Prog Ser 91:105–115
Stagg RM, Rusin J, Brown F (1992b) Na+, K+-ATPase activity in the gills of the flounder (Platichthys flesus) in relation to mercury contamination in the Firth of Forth. Mar Environ Res 33:255–266
ter Braak CJF, Prentice IC (1988) A theory of gradient analysis. Adv Ecol Res 18:271–317
Tietze F (1969) Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione. Anal Biochem 27:502–522
Torres MA, Testa CP, Gáspari C et al (2002) Oxidative stress in the mussel Mytella guyanensis from polluted mangroves on Santa Catarina Island, Brazil. Mar Pollut Bull 44:923–932
Towle DW, Holleland T (1987) Ammonium ion substitutes for K+ in ATP-dependent Na+ transport by basolateral membrane vesicles. Am J Physiol 252:R479–R489
van der Oost R, Goksoyr A, Celander M et al (1996a) Biomonitoring of aquatic pollution with feral eel (Anguilla anguilla) II. Biomarkers: pollution-induced biochemical responses. Aquat Toxicol 36:189–222
van der Oost R, Opperhuizen A, Satumalay K et al (1996b) Biomonitoring aquatic pollution with feral eel (Anguilla anguilla). I. Bioaccumulation: biota-sediment ratios of PCBs, OCPs, PCDDs abd PCDFs. Aquat Toxicol 35:21–46
van der Oost R, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13:57–149
Van Ginneken V, Van den Thillart GE (2000) Eel fat stores are enough to reach the Sargasso. Nature 403:156–157
van Ginneken V, Durif C, Balm SP et al (2007) Silvering of European eel (Anguilla anguilla L.): seasonal changes of morphological and metabolic parameters. Anim Biol 57:63–77
Vassault A (1983) Lactate dehydrogenase. In: Bergmeyer MO (ed) Methods of enzymatic analysis enzymes: oxireductases, transferases. Academic Press, New York, pp 118–126
Viarengo A, Betella E, Fabbri R et al (1997) Heavy metal inhibition of EROD activity in liver microsomes from the bass Dicentrarchus labrax exposed to organic xenobiotics: role of GSH in the reduction of heavy metal effects. Mar Environ Res 44:1–11
Watson TA, Beamish FWH (1980) Effects of zinc on branchial ATPase activity in vivo in rainbow trout, Salmo gairdneri. Comp Biochem Physiol C 66:77–82
Watson TA, Beamish FWH (1981) The effects of zinc on branchial adenosine triphosphatase enzymes in vitro from rainbow trout, Salmo gairdneri. Comp Biochem Physiol C 68:167–173
Wendelaar Bonga SE (1997) The stress response in fish. Physiol Rev 77:591–625
Wu RSS, Pollino CA, Au DWT et al (2003) Evaluation of biomarkers of exposure and effect in juvenile areolated grouper (Epinephelus areolatus) on foodborne exposure to benzo[a]pyrene. Environ Toxicol Chem 22:1568–1573
Acknowledgments
The authors thank Drs. C. Antunes, A. Oliveira and I. Cunha for their help during the capture of eels, and Dr. J. Wilson for the technical support provided on the Na+/K+-ATPase determination. Acknowledgments are also due to M. Faria, and A. Alves for technical assistance in some parts of the work. This study was funded by “Fundação para a Cíência e a Tecnologia” of Portugal (FCT), and by EU FEDER funds, through the project EELEANORA (POCTI/BSE/47918/2002) and a Post-Doc grant to C. Gravato (POCTI/BPD/21070/2004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guimarães, L., Gravato, C., Santos, J. et al. Yellow eel (Anguilla anguilla) development in NW Portuguese estuaries with different contamination levels. Ecotoxicology 18, 385–402 (2009). https://doi.org/10.1007/s10646-008-0294-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-008-0294-x