Abstract
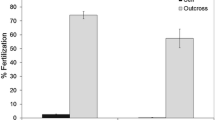
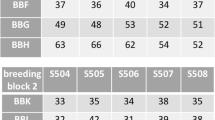
Genetic and ecological factors may interact in their effects on fitness. Such interactions are thus to be expected between inbreeding and exposure of a population to a toxicant. The magnitude of inbreeding depression is thought to increase in stressful environments. To test this hypothesis, we investigated the combined effects of environmental conditions and inbreeding on fitness in the self-fertile snail Lymnaea stagnalis, using a stress gradient (0–2) applied to a 100 isolated and paired lineages: laboratory control (0), outdoor microcosm control (1) and pesticide exposure under outdoor microcosm conditions (2). Outdoor stress conditions were maintained for 28 days prior to measurements of fitness traits (fecundity, hatching success, and size at hatching) under laboratory conditions, so that delayed environmental effects could be estimated. Under laboratory control conditions, we found significant initial family level heterogeneity for most measured traits, including physiological performances as assessed through energetic biomarkers. Whatever the environmental conditions, inbreeding depression was very low for progeny performances. Negative values of self-fertilization depression (SFD) were obtained. Unexpectedly, SFD showed a negative relationship with the assumed stress intensity, reflecting a higher sensitivity under pairing than under selfing, mostly due to parental fecundity. This suggests that stressful conditions may favour selfing. Stress intensity increased the distribution limits of both depression indices, suggesting that changes in fitness are less predictable in a population under stress. Implications of such findings for environmental risk assessment of pesticides are discussed.



Similar content being viewed by others
References
Aarssen L.W., (2000). Why are most selfers annuals? A new hypothesis for the fitness benefit of selfing Oikos 89:606–12
Barata C., Baird D.J., Minarro A., Soares A.M.V.M., (2000). Do genotype responses always converge from lethal to nonlethal toxicant exposure levels? Hypothesis tested using clones of Daphnia magna Strauss Environ. Toxicol. Chem. 19:2314–22
Baturo W., Lagadic L., Caquet T., (1995). Growth, fecundity and glycogen utilization in Lymnaea palustris exposed to atrazine and hexachlorobenzene in freshwater mesocosms Environ. Toxicol. Chem. 14:503–11
Beckerman A., Benton T.G., Ranta E., Kaitala V., Lundberg P., (2002). Population dynamic consequences of delayed life-history effects Trends Ecol. Evol. 17:263–9
Belfiore N.M., Anderson S.L., (2001). Effects of contaminants on genetic patterns in aquatic organisms: a review Mutat. Res. 489:97–122
Bickham J.W., Sandhu S., Hebert P.D.N., Chikhi L., Athwal R., (2000). Effects of chemicals on genetic diversity in natural populations: implications for biomonitoring and ecotoxicology Mutat. Res. 463:33–51
Bijlsma R., Bundgaard J., Van Putten W.F., (1999). Environmental dependence of inbreeding depression and purging in Drosophila melanogaster J. Evol. Biol. 12:1125–37
Byers D.L., Waller D.M., (1999). Do plant populations purge their genetic load? Effects of population size and mating history on inbreeding depression Ann. Rev. Ecol. Syst. 30:479–513
Charlesworth D., Charlesworth B., (1987). Inbreeding depression and its evolutionary consequences Ann. Rev. Ecol. Syst. 18:237–68
Cheptou P.O., Dieckmann U., (2002). The evolution of self-fertilization in density-regulated populations Proc. Roy. Soc. Lond. B 269:1177–86
Cheptou P.O., Imbert E., Lepart J., Escarre J., (2000). Effects of competition on lifetime estimates of inbreeding depression in the outcrossing plant Crepis sancta (Asteraceae) J. Evol. Biol. 13:522–31
Cheptou P.O., Mathias A., (2001). Can varying inbreeding depression select for intermediate selfing rates? Am. Nat. 157:361–73
Coutellec-Vreto M.A., Guiller A., Daguzan J., (1994). Allozyme variation in some populations of the freshwater snails Lymnaea peregra, L. auricularia and L. stagnalis (Gastropoda: Pulmonata) J. Moll. Stud. 60:393–403
Coutellec-Vreto M.A., Jarne P., Guiller A., Madec L., Daguzan J., (1998). Inbreeding and fitness in the freshwater snail Lymnaea peregra: an evaluation over two generations of self-fertilization Evolution 52:1635–47
Crawley M.J., (1993). GLIM for Ecologists. Blackwell Scientific Publications, Oxford
Crawley M.J., (2002). Statistical Computing, an Introduction to Data Analysis using S-plus. John Wiley and Sons, New York
Dahlgaard J., Hoffmann A.A., (2000). Stress resistance and environmental dependency of inbreeding depression in Drosophila melanogaster Cons. Biol. 14:1187–92
Dahlgaard J., Krebs R.A., Loeschke V., (1995). Heat-shock tolerance and inbreeding in Drosophila buzzati Heredity 78:410–6
Depledge M.H., (1994) Genotypic toxicity: implications for individuals and populations Environ. Health. Perspect. 102(Suppl 12):101–4
De Visser J.A., Ter Maat A., Zonneveld C., (1994). Energy budgets and reproductive allocation in the simultaneous hermaphrodite pond snail, Lymnaea stagnalis (L.): a trade-off between male and female function Am. Nat. 144:861–7
Doums C., Viard F., Pernot A.F., Delay B., Jarne P., (1996). Inbreeding depression, neutral polymorphism and copulatory behavior in freshwater snails: a self-fertilization syndrome Evolution 50:1908–18
D’surney S.J., Shugart L.R., Theodorakis C.W., (2001). Genetic markers and genotyping methodologies: an overview Ecotoxicology 10:201–4
Duivenboden Y.A. van, Pieneman A.W., Ter Maat A., (1985). Multiple mating suppresses fecundity in the hermaphrodite freshwater snail Lymnaea stagnalis: a laboratory study Anim. Behav. 33:1184–91
Forbes V.E., Depledge M.H., 1992. Predicting population response to pollutants: the significance of sex Funct. Ecol. 6:376–81
Forbes V.E., Moller V., Depledge M.H., (1995). Intrapopulation variability in sublethal response to heavy metal stress in sexual and asexual gastropod populations Funct. Ecol. 9:477–84
Fowler K., Whitlock M.C., (2002). Environmental stress, inbreeding, and the nature of phenotypic and genetic variance in Drosophila melanogasterProc. Roy. Soc. Lond. B 269:677–83
Guttman S.I., (1994). Population genetic structure and ecotoxicology Environ. Health Perspect. 102(Suppl 12): 97–100
Haag C.R., Sakwinska O., Ebert D., (2003). Test of synergistic interaction between infection and inbreeding in Daphnia magna Evolution 57:777–83
Hedrick P.W., (1994). Purging inbreeding depression and the probability of extinction: full-sib mating Heredity 73:363–72
Hedrick P.W., Kalinowski S.T., (2000). Inbreeding depression and conservation biology Ann. Rev. Ecol. Syst. 31:139–62
Henry P.Y., Pradel R., Jarne P., (2003). Environment-dependent inbreeding depression in a hermaphroditic freshwater snail J. Evol. Biol. 16:1211–22
Hoffmann A.A., Parsons P.A., (1997). Extreme Environmental Change and Evolution. Cambridge University Press, Cambridge
Holloway G.J., Crocker H.J., Callaghan A., (1997). The effects of novel and stressful environments on trait distribution Funct. Ecol. 11:579–84
Jarne P., Charlesworth D., (1993). The evolution of the selfing rate in functionally hermaphrodite plants and animals Ann. Rev. Ecol. Syst. 24:441–66
Jarne P., Finot L., Bellec C., Delay B., (1992). Aphally versus euphally in self-fertile hermaphrodite snails from the species Bulinus truncatus (Pulmonata: Planorbidae) Am. Nat. 139:424–32
Jarne P., Finot L., Delay B., Thaler L., (1991). Self-fertilization versus cross-fertilization in the hermaphroditic freshwater snail Bulinus globosus Evolution 45:1136–46
Jarne P., Perdieu M.A., Pernot A.F., Delay B., David P., (2000). The influence of self-fertilization and grouping on fitness attributes in the freshwater snail Physa acuta: population and individual inbreeding depression J. Evol. Biol. 13:645–55
Jumel A., Coutellec M.A., Cravedi J.P., Lagadic L., (2002). Nonylphenol polyethoxylate adjuvant mitigates the reproductive toxicity of fomesafen on the freshwater snail Lymnaea stagnalis in outdoor experimental ponds Environ. Toxicol. Chem. 21:1876–88
Kammenga J., Laskowski R., (2000). Demographic approaches in ecotoxicology: state of the art In Kammenga J., Laskowski R., (eds.) Demography in Ecotoxicology, John Wiley and Sons New York pp.3–8
Keller L.F., Grant P.R., Grant R., Petren K., (2002). Environmental conditions affect the magnitude of inbreeding depression in survival of Darwin’s finches Evolution 56:1229–39
Knott E., Puurtinen M., Kaitala V., (2003). Primers for nine microsatellite loci in the hermaphroditic snail Lymnaea stagnalis Mol. Ecol. Notes 3:333–5
Koene J.M., Ter Maat A.T., (2005). Sex role alternation in the simultaneous hermaphroditic pond snail Lymnaea stagnalis is determined by the availability of seminal fluid Anim. Behav. 69:845–50
Lande R., Schemske D.W., (1985). The evolution of self-fertilization and inbreeding depression in plants I. Genetic modelsEvolution 39:24–40
Lande R., Schemske D.W., Schultz S.T., (1994). High inbreeding depression, selective interference among loci, and the threshold selfing rate for purging recessive lethal mutations Evolution 48:965–78
Loeschcke V., Krebs R.A., Dahlgaard J., Michalak P., (1997). High-temperature stress and the evolution of thermal resistance in Drosophila. In Bilsjma R., Loeschcke V., (eds.) Environmental Stress, Adaptation and Evolution, Birkhäuser Verlag Basel, pp. 175–90
Lynch M., (1988). Design and analysis of experiments on random drift and inbreeding depressionGenetics 120:791–807
Lynch M., Blanchard D., Houle D., Kibota T., Schultz S., Vassilieva L., Willis J., (1999). Perspective: spontaneous deleterious mutation Evolution 53:645–63
Mahendru V.K., Agarwal R.A., (1981). Changes in carbohydrate metabolism in various organs of the snail, Lymnaea acuminata following exposure to trichlorfon Acta Pharmacol. Toxicol. 48:377–81
McCullagh P., Nelder J.A., (1983). Generalized Linear Models. Chapman and Hall London
Miller P.S., (1994). Is inbreeding depression more severe in stressful environments? Zool. Biol. 13:195–208
Mousseau T.A., Fox C.W., (1998). The adaptive significance of maternal effects Trends Ecol. Evol. 13:403–7
Nason J.D., Ellstrand N.C., (1995). Lifetime estimates of biparental inbreeding depression in the self-incompatible annual plant Raphanus sativus Evolution 49:307–16
Pray L.A., Schwartz J.M., Goodnight C.J., Stevens L., (1994). Environmental dependency of inbreeding depression: implications for conservation biology Conserv. Biol. 8:562–8
Puurtinen M., Knott K.E., Suonpää S., van Ooik T., Kaitala V., (2004). Genetic variability and drift load in populations of an aquatic snail Evolution 58:749–56
Puurtinen, H.M., Suonpää, S., Nissinen, K. and Kaitala, V. (2001). The relationship between genetic load and inbreeding depression as revealed by snail populations. Eighth Congress of the European Society for Evolutionary Biology, 20–25 August 2001. Aarhus, Denmark. Abstracts, p. 152
Simmons M.J., Crow J.F., (1977). Mutations affecting fitness in Drosophila populations Ann. Rev. Genetics 11:49–78
Snell T.W., Serra M., Carmona M.J., (1998). Toxicity and sexual reproduction in rotifers: reduced resting egg production and heterozygosity loss. In Forbes V.E., (eds.) Genetics and Ecotoxicology, Taylor and Francis, Philadelphia, pp.169–85
Theodorakis C.W., (2001). Integration of genotoxic and population genetic endpoints in biomonitoring and risk assessment Ecotoxicology 10:245–56
Theodorakis C.W., Shugart L.R., (1997). Genetic ecotoxicology II: population genetic structure in mosquitofish exposed in situ to radionuclides Ecotoxicology 6:335–54
Tsitrone A., Duperron S., David P., (2003). Delayed selfing as an optimal mating strategy in preferentially outcrossing species: theoretical analysis of the optimal age at first reproduction in relation to mate availability Am. Nat. 162:318–31
Uyenoyama M.K., Holsinger K.E., Waller D.M., (1993). Ecological and genetic factors directing the evolution of self-fertilization Oxf. Surv. Evol. Biol. 9:327–81
Wiehn J., Kopp K., Rezzonico S., Karttunen S., Jokela J., (2002). Family-level covariation between parasite resistance and mating system in a hermaphroditic freshwater snail Evolution 56:1454–61
Acknowledgments
We thank D. Azam and A. Quemeneur (Experimental Unit of Aquatic Ecology and Ecotoxicology, INRA, Rennes, France) for maintenance of snail lineages and microcosm settling, and M. Heydorff for biochemical measurements. We also thank Ph. Jarne and Th. Caquet for valuable discussions and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Coutellec, MA., Lagadic, L. Effects of Self-Fertilization, Environmental Stress and Exposure to Xenobiotics on Fitness-Related Traits of the Freshwater Snail Lymnaea stagnalis . Ecotoxicology 15, 199–213 (2006). https://doi.org/10.1007/s10646-005-0049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10646-005-0049-x