Summary
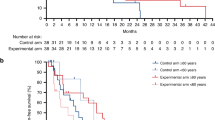
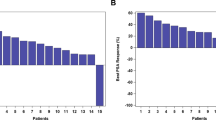
C-X-C motif chemokine receptor 2 (CXCR2) has a role in tumor progression, lineage plasticity, and reduction of immune checkpoint inhibitor efficacy. Preclinical evidence suggests potential benefit of CXCR2 inhibition in multiple solid tumors. In this phase 2 study (NCT03473925), adults with previously treated advanced or metastatic castration-resistant prostate cancer (CRPC), microsatellite-stable colorectal cancer (MSS CRC), or non–small-cell lung cancer (NSCLC) were randomized 1:1 to the CXCR2 antagonist navarixin 30 or 100 mg orally once daily plus pembrolizumab 200 mg intravenously every 3 weeks up to 35 cycles. Primary endpoints were investigator-assessed objective response rate (RECIST v1.1) and safety. Of 105 patients (CRPC, n=40; MSS CRC, n=40; NSCLC, n=25), 3 had a partial response (2 CRPC, 1 MSS CRC) for ORRs of 5%, 2.5%, and 0%, respectively. Median progression-free survival was 1.8–2.4 months without evidence of a dose-response relationship, and the study was closed at a prespecified interim analysis for lack of efficacy. Dose-limiting toxicities occurred in 2/48 patients (4%) receiving navarixin 30 mg and 3/48 (6%) receiving navarixin 100 mg; events included grade 4 neutropenia and grade 3 transaminase elevation, hepatitis, and pneumonitis. Treatment-related adverse events occurred in 70/105 patients (67%) and led to treatment discontinuation in 7/105 (7%). Maximal reductions from baseline in absolute neutrophil count were 44.5%−48.2% (cycle 1) and 37.5%−44.2% (cycle 2) and occurred within 6−12 hours postdose in both groups. Navarixin plus pembrolizumab did not demonstrate sufficient efficacy in this study. Safety and tolerability of the combination were manageable. (Trial registration: ClinicalTrials.gov, NCT03473925).



Similar content being viewed by others
Data availability
Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Applications will be promptly assessed for completeness and policy compliance. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.
References
Cheng Y, Ma XL, Wei YQ, Wei XW (2019) Potential roles and targeted therapy of the CXCLs/CXCR2 axis in cancer and inflammatory diseases. Biochim Biophys Acta Rev Cancer 1871:289–312. https://doi.org/10.1016/j.bbcan.2019.01.005
Korbecki J, Kupnicka P, Chlubek M, Goracy J, Gutowska I, Baranowska-Bosiacka I (2022) CXCR2 receptor: regulation of expression, signal transduction, and involvement in cancer. Int J Mol Sci 23:2168 [Epub]. https://doi.org/10.3390/ijms23042168
Di Mitri D, Mirenda M, Vasilevska J, Calcinotto A, Delaleu N, Revandkar A, Gil V, Boysen G, Losa M et al (2019) Re-education of tumor-associated macrophages by CXCR2 blockade drives senescence and tumor inhibition in advanced prostate cancer. Cell Rep 28(2156–2168):e2155. https://doi.org/10.1016/j.celrep.2019.07.068
Zhao J, Ou B, Feng H, Wang P, Yin S, Zhu C, Wang S, Chen C, Zheng M et al (2017) Overexpression of CXCR2 predicts poor prognosis in patients with colorectal cancer. Oncotarget 8:28442–28454. https://doi.org/10.18632/oncotarget.16086
Wei L, Liu Y, Ma Y, Ding C, Zhang H, Lu Z, Gu Z, Zhu C (2019) C-X-C chemokine receptor 2 correlates with unfavorable prognosis and facilitates malignant cell activities via activating JAK2/STAT3 pathway in non-small cell lung cancer. Cell Cycle 18:3456–3471. https://doi.org/10.1080/15384101.2019.1689471
Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, Zhang H, Zhou Y, Gao AC et al (2019) Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci Transl Med 11:eaax0428. https://doi.org/10.1126/scitranslmed.aax0428
Condamine T, Ramachandran I, Youn JI, Gabrilovich DI (2015) Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 66:97–110. https://doi.org/10.1146/annurev-med-051013-052304
Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M et al (2018) IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature 559:363–369. https://doi.org/10.1038/s41586-018-0266-0
Li T, Liu T, Zhu W, Xie S, Zhao Z, Feng B, Guo H, Yang R (2021) Targeting MDSC for immune-checkpoint blockade in cancer immunotherapy: current progress and new prospects. Clin Med Insights Oncol 15:11795549211035540. https://doi.org/10.1177/11795549211035540
Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, Watz H, Lu S, Stryszak P et al (2015) CXCR2 antagonist MK-7123. A phase 2 proof-of-concept trial for chronic obstructive pulmonary disease. Am J Respir Crit Care Med 191:1001–1011. https://doi.org/10.1164/rccm.201405-0992OC
Nair P, Gaga M, Zervas E, Alagha K, Hargreave FE, O’Byrne PM, Stryszak P, Gann L, Sadeh J et al (2012) Safety and efficacy of a CXCR2 antagonist in patients with severe asthma and sputum neutrophils: a randomized, placebo-controlled clinical trial. Clin Exp Allergy 42:1097–1103. https://doi.org/10.1111/j.1365-2222.2012.04014.x
Jasper AE, McIver WJ, Sapey E, Walton GM (2019) Understanding the role of neutrophils in chronic inflammatory airway disease. F1000Res 8:557. https://doi.org/10.12688/f1000research.18411.1
Ning Y, Labonte MJ, Zhang W, Bohanes PO, Gerger A, Yang D, Benhaim L, Paez D, Rosenberg DO et al (2012) The CXCR2 antagonist, SCH-527123, shows antitumor activity and sensitizes cells to oxaliplatin in preclinical colon cancer models. Mol Cancer Ther 11:1353–1364. https://doi.org/10.1158/1535-7163.MCT-11-0915
Singh S, Sadanandam A, Nannuru KC, Varney ML, Mayer-Ezell R, Bond R, Singh RK (2009) Small-molecule antagonists for CXCR2 and CXCR1 inhibit human melanoma growth by decreasing tumor cell proliferation, survival, and angiogenesis. Clin Cancer Res 15:2380–2386. https://doi.org/10.1158/1078-0432.CCR-08-2387
Fu S, Lin J (2018) Blocking interleukin-6 and interleukin-8 signaling inhibits cell viability, colony-forming activity, and cell migration in human triple-negative breast cancer and pancreatic cancer cells. Anticancer Res 38:6271–6279. https://doi.org/10.21873/anticanres.12983
Fu S, Chen X, Lin HJ, Lin J (2018) Inhibition of interleukin 8/CX-C chemokine receptor 1,/2 signaling reduces malignant features in human pancreatic cancer cells. Int J Oncol 53:349–357. https://doi.org/10.3892/ijo.2018.4389
Varney ML, Singh S, Li A, Mayer-Ezell R, Bond R, Singh RK (2011) Small molecule antagonists for CXCR2 and CXCR1 inhibit human colon cancer liver metastases. Cancer Lett 300:180–188. https://doi.org/10.1016/j.canlet.2010.10.004
Zeng Y, Li B, Liang Y, Reeves PM, Qu X, Ran C, Liu Q, Callahan MV, Sluder AE et al (2019) Dual blockade of CXCL12-CXCR4 and PD-1-PD-L1 pathways prolongs survival of ovarian tumor-bearing mice by prevention of immunosuppression in the tumor microenvironment. FASEB J 33:6596–6608. https://doi.org/10.1096/fj.201802067RR
Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, Das R, Silvin C, Van Waes C et al (2019) Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight 4:e126853. https://doi.org/10.1172/jci.insight.126853
Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, Foth M, Bryson S, McDaid K et al (2016) CXCR2 inhibition profoundly suppresses metastases and augments immunotherapy in pancreatic ductal adenocarcinoma. Cancer Cell 29:832–845. https://doi.org/10.1016/j.ccell.2016.04.014
Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS et al (2015) PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 372:2509–2520. https://doi.org/10.1056/NEJMoa1500596
Antonarakis ES, Piulats JM, Gross-Goupil M, Goh J, Ojamaa K, Hoimes CJ, Vaishampayan U, Berger R, Sezer A et al (2020) Pembrolizumab for treatment-refractory metastatic castration-resistant prostate cancer: multicohort, open-label phase II KEYNOTE-199 study. J Clin Oncol 38:395–405. https://doi.org/10.1200/JCO.19.01638
Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M et al (2015) Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372:2018–2028. https://doi.org/10.1056/NEJMoa1501824
Garon EB, Hellmann MD, Rizvi NA, Carcereny E, Leighl NB, Ahn MJ, Eder JP, Balmanoukian AS, Aggarwal C et al (2019) Five-year overall survival for patients with advanced non-small-cell lung cancer treated with pembrolizumab: results from the phase I KEYNOTE-001 study. J Clin Oncol 37:2518–2527. https://doi.org/10.1200/JCO.19.00934
Hastrup N, Khalilieh S, Dale DC, Hanson LG, Magnusson P, Tzontcheva A, Tseng J, Huyck S, Rosenberg E et al (2015) The effects of the CXCR2 antagonist, MK-7123, on bone marrow functions in healthy subjects. Cytokine 72:197–203. https://doi.org/10.1016/j.cyto.2015.01.002
Todd CM, Salter BM, Murphy DM, Watson RM, Howie KJ, Milot J, Sadeh J, Boulet LP, O’Byrne PM et al (2016) The effects of a CXCR1/CXCR2 antagonist on neutrophil migration in mild atopic asthmatic subjects. Pulm Pharmacol Ther 41:34–39. https://doi.org/10.1016/j.pupt.2016.09.005
Guo C, Sharp A, Gurel B, Crespo M, Figueiredo I, Jain S, Vogl U, Rekowski J, Rouhifard M et al (2023) Targeting myeloid chemotaxis to reverse prostate cancer therapy resistance. Nature 623:1053–1061. https://doi.org/10.1038/s41586-023-06696-z
Cohen EE, Hong DS, Wise Draper T, Nassib William W, Schrijvers D, Mesia Nin R, Scott ML, Lyne P, Mugundu G et al (2017) Phase 1b/2 Study (SCORES) assessing safety, tolerability, and preliminary anti-tumor activity of durvalumab plus AZD9150 or AZD5069 in patients with advanced solid malignancies and squamous cell carcinoma of the head and neck (SCCHN) [abstract]. Ann Oncol 28:V403. https://doi.org/10.1093/annonc/mdx376.001
ClinicalTrials.gov. Study to assess MEDI4736 with either AZD9150 or AZD5069 in advanced solid tumors & relapsed metastatic squamous cell carcinoma of head & neck (NCT02499328). Available at https://clinicaltrials.gov/ct2/show/NCT02499328. Accessed September 1, 2022
ClinicalTrials.gov. Phase Ib/II study of MEDI4736 evaluated in different combinations in metastatic pancreatic ductal carcinoma (NCT02583477). Available at https://clinicaltrials.gov/ct2/show/NCT02583477. Accessed September 1, 2022
ClinicalTrials.gov. Combination study of AZD5069 and enzalutamide. (ACE) (NCT03177187). Available at https://clinicaltrials.gov/ct2/show/NCT03177187. Accessed September 1, 2022
Guo C, Sharp A, Vogl U, Colombo I, Stathis A, Jain S, Chandran K, Tiu C, Paschalis A et al (2022) A phase (Ph) I/II trial of the CXCR2 antagonist AZD5069 in combination with enzalutamide (ENZA) in patients (pts) with metastatic castration resistant prostate cancer (mCRPC). Ann Oncol 33:S197–S224. https://doi.org/10.1016/annonc/annonc1049
Goldstein LJ, Perez RP, Yardley D, Han LK, Reuben JM, Gao H, McCanna S, Butler B, Ruffini PA et al (2020) A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res 22:4 [Epub]. https://doi.org/10.1186/s13058-019-1243-8
Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, Perez RP, Kato G, Wicha M (2017) Phase Ib pilot study to evaluate reparixin in combination with weekly paclitaxel in patients with HER-2-negative metastatic breast cancer. Clin Cancer Res 23:5358–5365. https://doi.org/10.1158/1078-0432.CCR-16-2748
Goldstein LJ, Mansutti M, Levy C, Chang JC, Henry S, Fernandez-Perez I, Prausova J, Staroslawska E, Viale G et al (2021) A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res Treat 190:265–275. https://doi.org/10.1007/s10549-021-06367-5
Acknowledgements
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. We thank the patients and their families and caregivers for participating in this study, along with all investigators, the co-investigators Katherine Scilla, MD, and Ranee Mehra, MD, and site personnel. Medical writing assistance was provided by Autumn Kelly, MA, of ICON plc (Blue Bell, PA, USA). This assistance was funded by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA. Dr. Armstrong reports funding from the NIH through 1R01CA233585-04.
Funding
Funding for this research was provided by Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA.
Author information
Authors and Affiliations
Contributions
Conceptualization: Andrew J. Armstrong, Vincent Giranda, Fang Liu, and Bhargava Kandala. Methodology: Andrew J. Armstrong, Vincent Giranda, Fang Liu, and Bhargava Kandala. Formal analysis and investigation: Andrew J. Armstrong, Ravit Geva, Hyun Cheol Chung, Charlotte Lemech, Wilson H. Miller Jr., Aaron R. Hansen, Jong-Seok Lee, Frank Tsai, Benjamin J. Solomon, Tae Min Kim, Christian Rolfo, Vincent Giranda, Yixin Ren, Fang Liu, Bhargava Kandala, Tomoko Freshwater, and Judy S. Wang. Writing – original draft preparation: Andrew J. Armstrong, Hyun Cheol Chung, Frank Tsai, and Vincent Giranda. Writing – review and editing: Andrew J. Armstrong, Ravit Geva, Hyun Cheol Chung, Charlotte Lemech, Wilson H. Miller Jr., Aaron R. Hansen, Jong-Seok Lee, Benjamin J. Solomon, Tae Min Kim, Christian Rolfo, Vincent Giranda, Yixin Ren, Fang Liu, Bhargava Kandala, Tomoko Freshwater, and Judy S. Wang. Resources: Andrew J. Armstrong, Hyun Cheol Chung, Wilson H. Miller Jr., and Aaron R. Hansen.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with local and/or national regulations and with the Declaration of Helsinki. The study protocol was approved by the institutional review board or independent ethics committee at each site.
Consent
Informed consent was obtained from all patients included in the study.
Competing interests
Andrew J. Armstrong: Research support (to Duke) from Astellas, Pfizer, Bayer, Janssen, Dendreon, Genentech/Roche, Bristol Myers Squibb, AstraZeneca, Merck, Forma, Celgene, and Amgen. Consulting or advising relationships with Astellas, Epic Sciences, Pfizer, Bayer, Janssen, Dendreon, Bristol Myers Squibb, AstraZeneca, Merck, Forma, Celgene, Clovis, Exact Sciences, Myovant, Exelixis, Point Biopharma, Medscape CME, OncLive, and Research to Practice. Ravit Geva: Options: Pyxis. Medical Lead: Pyxis. Honoraria: Roche, Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck & Co., Inc., Rahway, NJ, USA, Medison, Janssen, Pfizer, and Bristol Myers Squibb. Consulting and Advisory: Eisai, AstraZeneca, Roche, Ranium, Johnson & Johnson, Bayer, Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and Oncotest. Travel, Accommodations, Expenses: Takeda and Medison. Hyun Cheol Chung: Grants/Research Support: Lilly, GSK, Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Merck-Serono, Bristol Myers Squibb/Ono, Taiho, Amgen, BeiGene, Incyte, and Zymeworks. Honoraria: Merck-Serono and Lilly. Consultation: Taiho, Celltrion, Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, Lilly, Bristol Myers Squibb, Merck-Serono, Gloria, BeiGene, Amgen, and Zymeworks. Charlotte Lemech: No competing interests to disclose. Wilson H. Miller Jr.: Grants Support: Merck, CIHR, CRS, Terry Fox Research Institute, Samuel Waxman Cancer Research Foundation, and CCSRI. Participated in clinical trials within the past 2 years: Bristol Myers Squibb, Novartis, GSK, Roche, AstraZeneca, Methylgene, MedImmune, Bayer, Amgen, Merck, Incyte, Pfizer, Sanofi, Array, MiMic, Ocellaris Pharma, Astellas, Alkermes, Exelixis, VelosBio, and Genentech. Consulting/Honoraria: Merck, Bristol Myers Squibb, Roche, GSK, Novartis, Amgen, Mylan, EMD Serono, and Sanofi. Aaron R. Hansen: Research: Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, GSK, Bristol Myers Squibb, Novartis, Pfizer, Roche, Genentech, Boehringer Ingelheim, Tyra Biosciences, Neoleukin Therapeutics, Astellas, Point Biopharma, and AstraZeneca. Consulting: Pfizer, Merck, and GSK. Jong-Seok Lee: No competing interests to disclose. Frank Tsai: No competing interests to disclose. Benjamin J. Solomon: Advisory Boards/Honoraria: Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, AstraZeneca, Pfizer, Novartis, Takeda, Bristol Myers Squibb, Eli Lilly, Novartis, Amgen, BeiGene, and Roche. Tae Min Kim: Received honoraria from or played an advisory role with AstraZeneca, Boryung, F. Hoffmann-La Roche Ltd/Genentech, Inc., IMBDx, Inc., Janssen, Novartis, Regeneron, Samsung Bioepis, Sanofi, Takeda, and Yuhan, and has received research funding outside this work from AstraZeneca-Korea Health Industry Development Institute. Christian Rolfo: Consulting fees: Archer, Inivata, Bristol Myers Squibb, Novartis, Boston Pharmaceuticals, EMD Serono, Pfizer, Mirati, Eisai, Daiichi Sankyo, Sanofi Genzyme-Regeneron, Blueprint Medicines, CEA, Bayer U.C. LLC, General Dynamics, MedStar, Diverse HealthHub, Merck, MH Live Events, Janssen Scientific Affairs, EMD, Amgen, LUNGevity, Postgraduate Institute of Medicine, ACC Med, GME, Nadirex, ASCO Plenary Series, Imagene, HMP Education, Medical Educator Consortium, Thermo Fisher Scientific, and AbbVie. Research grant: LCRF-Pfizer. Ownership interest: Novartis. Fees for non-CME/CE services: AstraZeneca, Roche, Guardant Health, Merck Sharp and Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, COR2ED, Physicians Education Resource, LLC, Intellisphere, LLC, and Boehringer Ingelheim. Participation on a Data Safety Monitoring Board: EMD Serono. President: ISLB. Chair Educational Committee: IASLC. Scientific Board Member: ESO. Faculty Lung Cancer Advanced Editor in Chief: CROH. Associate Editor: ESMO Open. Vincent Giranda: former employee of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stockholder in Merck & Co., Inc., Rahway, NJ, USA. Yixin Ren, Fang Liu, Bhargava Kandala, and Tomoko Freshwater: employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stockholders in Merck & Co., Inc., Rahway, NJ, USA. Judy S. Wang: Consulting/Advisory: Janssen Research & Development, Stemline Therapeutics, Kanaph Therapeutics, and Fusion Pharmaceuticals. Speakers’ Bureau: AstraZeneca and Eisai. Research Funding (to institution): AstraZeneca, Bicycle Therapeutics, BioNTech, Boehringer Ingelheim, Celgene, cyteir, Daiichi Sankyo, Genentech/Roche, GlaxoSmithKline, H3 Biomedicine, Hutchison MediPharma, Jacobio, Janssen Research & Development, Klus Pharma, Kymab, Loxo, LSK BioPharma, Macrogenics, Merck, Moderna Therapeutics, Phoenix Pharmaceuticals, Prelude Therapeutics, QiLu Pharmaceutical, Revolution Medicines, Ribon Therapeutics, Syndax, Taiho Pharmaceutical, Tesaro, TopAlliance BioSciences Inc, Xencor, Artios, Erasca, Inc, Immunogen, Cullinan Oncology, Immune-Onc Therapeutics, Bayer Health, Biosplice, Zymeworks, BioTheryX, TeneoBio, Nurix, IgM Biosciences, PureTech, Forty Seven, Treadwell Therapeutics, MabSpace Biosciences, Novartis, Olema Oncology, Seven and Eight Biopharmaceuticals, ORIC Pharmaceuticals, Relay Therapeutics, Jazz Pharmaceuticals, Adagene, NGM Biopharmaceuticals, Agenus, Metabomed, BeiGene, Astellas Pharma, Blueprint Medicines, and Hotspot Therapeutics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Vincent Giranda is now retired.
Christian Rolfo affiliation at time of study: Greenebaum Comprehensive Cancer Center, University of Maryland, Baltimore, MD, USA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Armstrong, A.J., Geva, R., Chung, H.C. et al. CXCR2 antagonist navarixin in combination with pembrolizumab in select advanced solid tumors: a phase 2 randomized trial. Invest New Drugs 42, 145–159 (2024). https://doi.org/10.1007/s10637-023-01410-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01410-2