Abstract
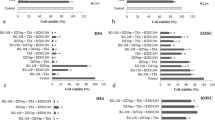
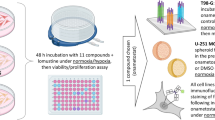
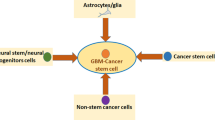
Glioblastomas (GBM), also known as glioblastoma multiforme, are the most aggressive type of brain cancer. Currently, there is no effective treatment for GBM, highlighting the pressing need for new therapeutic strategies. In a recent study, we demonstrated that specific combinations of epigenetic modifiers significantly affect the metabolism and proliferation rate of the two most aggressive GBM cell lines, D54 and U-87. Importantly, these combinations exhibited minimal effects on the growth of normal stem cells. In this study, we extended our investigation to include a patient-derived GBM stem cell line. Our results showed that the combinations of modulators of histone and DNA covalent modifying enzymes that synergistically suppress D54 and U87 cell line growth also impair the viability of the patient-derived GBM stem cell line. These findings suggest that epigenetic modifiers alone or in specific combinations exhibit a cytotoxic effect on established and low-passage patient-derived GBM cell lines, and thus could be a promising therapeutic approach for this type of brain cancer.


Similar content being viewed by others
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Carlsson SK, Brothers SP, Wahlestedt C (2014) Emerging treatment strategies for glioblastoma multiforme. EMBO Mol Med 6(11):1359–1370. https://doi.org/10.15252/emmm.201302627
da Hora CC, Schweiger MW, Wurdinger T, Tannous BA (2019) Patient-derived glioma models: from patients to dish to animals. Cells 8(10). https://doi.org/10.3390/cells8101177
Davis ME (2016) Glioblastoma: overview of Disease and Treatment.Clin. J Oncol Nurs 20(5 Suppl):S2–8. https://doi.org/10.1188/16.CJON.S1.2-8
Di Renzo MF, Corso S (2020) Patient-derived Cancer Models. Cancers (Basel) 12(12). https://doi.org/10.3390/cancers12123779
Li H, Lei B, Xiang W, Wang H, Feng W, Liu Y et al (2017) Differences in protein expression between the U251 and U87 cell Lines.Turk Neurosurg. 27(6):894–903. https://doi.org/10.5137/1019-5149.JTN.17746-16.1
Alexanian AR, Huang YW (2015) Specific combinations of the chromatin-modifying enzyme modulators significantly attenuate glioblastoma cell proliferation and viability while exerting minimal effect on normal adult stem cells growth.Tumour Biol. https://doi.org/10.1007/s13277-015-3654-1
Arthurs AL, Keating DJ, Stringer BW, Conn SJ (2020) The suitability of Glioblastoma Cell Lines as Models for primary glioblastoma cell Metabolism.Cancers (Basel). 12(12). https://doi.org/10.3390/cancers12123722
Ye LF, Reznik E, Korn JM, Lin F, Yang G, Malesky K et al (2020) Patient-derived glioblastoma cultures as a tool for small-molecule drug discovery.Oncotarget.11. 4443–451. https://doi.org/10.18632/oncotarget.27457
Goodspeed A, Heiser LM, Gray JW, Costello JC (2016) Tumor-derived cell lines as Molecular Models of Cancer Pharmacogenomics. Mol Cancer Res 14(1):3–13. https://doi.org/10.1158/1541-7786.MCR-15-0189
Sharma SV, Haber DA, Settleman J (2010) Cell line-based platforms to evaluate the therapeutic efficacy of candidate anticancer agents. Nat Rev Cancer 10(4):241–253. https://doi.org/10.1038/nrc2820
Stringer BW, Day BW, D’Souza RCJ, Jamieson PR, Ensbey KS, Bruce ZC et al (2019) A reference collection of patient-derived cell line and xenograft models of proneural, classical and mesenchymal glioblastoma. Sci Rep 9(1):4902. https://doi.org/10.1038/s41598-019-41277-z
Alexanian AR, Brannon A (2022) Unique combinations of epigenetic modifiers synergistically impair the viability of the U87 glioblastoma cell line while exhibiting minor or moderate effects on normal stem cell growth. Med Oncol 39(5):86. https://doi.org/10.1007/s12032-022-01683-2
Chaichana KL, Guerrero-Cazares H, Capilla-Gonzalez V, Zamora-Berridi G, Achanta P, Gonzalez-Perez O et al (2009) Intra-operatively obtained human tissue: protocols and techniques for the study of neural stem cells. J Neurosci Methods 180(1):116–125. https://doi.org/10.1016/j.jneumeth.2009.02.014
Binder ZA, Wilson KM, Salmasi V, Orr BA, Eberhart CG, Siu IM et al (2016) Establishment and Biological characterization of a panel of Glioblastoma Multiforme (GBM) and GBM variant Oncosphere Cell Lines. PLoS ONE 11(3):e0150271. https://doi.org/10.1371/journal.pone.0150271
Ying M, Wang S, Sang Y, Sun P, Lal B, Goodwin CR et al (2011) Regulation of glioblastoma stem cells by retinoic acid: role for notch pathway inhibition.Oncogene.30. 313454–3467. https://doi.org/10.1038/onc.2011.58
Li Y, Li A, Glas M, Lal B, Ying M, Sang Y et al (2011) c-Met signaling induces a reprogramming network and supports the glioblastoma stem-like phenotype. Proc Natl Acad Sci U S A 108(24):9951–9956. https://doi.org/10.1073/pnas.1016912108
Tilghman J, Wu H, Sang Y, Shi X, Guerrero-Cazares H, Quinones-Hinojosa A et al (2014) HMMR maintains the stemness and tumorigenicity of glioblastoma stem-like cells. Cancer Res 74(11):3168–3179. https://doi.org/10.1158/0008-5472.CAN-13-2103
Auvergne R, Wu C, Connell A, Au S, Cornwell A, Osipovitch M et al (2016) PAR1 inhibition suppresses the self-renewal and growth of A2B5-defined glioma progenitor cells and their derived gliomas in vivo.Oncogene.35. 293817–3828. https://doi.org/10.1038/onc.2015.452
Garcia CA, Bhargav AG, Brooks M, Suarez-Meade P, Mondal SK, Zarco N et al (2021) Functional characterization of brain tumor-initiating cells and establishment of GBM Preclinical Models that incorporate heterogeneity, therapy, and sex differences. Mol Cancer Ther 20(12):2585–2597. https://doi.org/10.1158/1535-7163.MCT-20-0547
Acknowledgements
This work was supported by Cell Reprogramming & Therapeutics LLC and partially by the support of National Institute of Health (NIH) grant 1 R43 CA221490.
Funding
This work was supported by Cell Reprogramming & Therapeutics LLC and partially by the support of National Institute of Health (NIH) grant 1 R43 CA221490
Author information
Authors and Affiliations
Contributions
Dr Alexanian contributed to study motivation, experimental design and writing the paper; Heidi Stoellinger contribution – technical support; Virginea De Araujo Farias and Alfredo Quinones-Hinojosa contribution – generation of patient derived cell line and with technical support for cells growth and expansion. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
No human or animal subjects involved. This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of cell Reprogramming & Therapeutics LLC and Mayo Clinic.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Alexanian, A.R., Stoellinger, H.M., de Araujo Farias, V. et al. Epigenetic modifiers either individually or in specific combinations impair viability of patient-derived glioblastoma cell line while exhibit moderate effect on normal stem cells growth. Invest New Drugs 41, 371–375 (2023). https://doi.org/10.1007/s10637-023-01370-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01370-7