Abstract
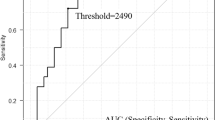
Soluble interleukin-2 receptor (sIL-2R) suppresses effector T-cells. Few studies have assessed serum sIL-2R in patients receiving immunotherapy. We evaluated the association between serum sIL-2R levels and the efficacy of anti-programmed cell death 1/ programmed death-ligand 1 (anti-PD-1/PD-L1) antibody combined with chemotherapy in non-small cell lung cancer (NSCLC) patients. We prospectively enrolled NSCLC patients who received anti-PD-1/PD-L1 antibody combined with platinum-based chemotherapy between 8/2019 and 8/2020 and measured their serum sIL-2R. The patients were divided into high and low sIL-2R groups based on the median of sIL-2R levels at pretreatment. Progression-free survival (PFS) and overall survival (OS) of patients in the high and low sIL-2R groups were compared. The Kaplan–Meier curves of PFS and OS were evaluated using the log-rank test. The multivariate analysis of PFS and OS was performed using the Cox proportional hazard models. Among 54 patients (median age 65, range 34–84), 39 were male and 43 had non-squamous cell carcinoma. The sIL-2R cut-off value was 533 U/mL. Median PFS was 5.1 months (95% CI, 1.8–7.5 months) and 10.1 months (95% CI, 8.3-not reached [NR] months) in the high and low sIL-2R groups (P = 0.007), respectively. Median OS was 10.3 months (95% CI, 4.0–NR months) and NR (95% CI, 10.3–NR months) in the high and low sIL-2R groups (P = 0.005), respectively. Multivariate Cox regression analysis showed that high sIL-2R was significantly associated with shorter PFS and OS. SIL-2R may be a biomarker for the poor efficacy of anti-PD-1/PD-L1 antibody combined with chemotherapy.



Similar content being viewed by others
Data Availability
The datasets in this study are available from the corresponding author on reasonable request.
Abbreviations
- ALK:
-
anaplastic lymphoma kinase rearrangement
- CI:
-
confidence intervals
- EGFR:
-
Epidermal growth factor receptor
- HR:
-
Hazard ratio
- PD-L1:
-
programmed cell death-ligand 1
- PS:
-
performance status
- S-IL2R:
-
soluble interleukin 2 receptor
- Sq:
-
squamous cell carcinoma
- TPS:
-
tumor proportion score
References
Siegel RL, Miller KD, Jemal A (2019) Cancer statistics, 2019. CA Cancer J Clin 69(1):7–34
Sher T, Dy GK, Adjei AA (2008) Small cell lung cancer. Mayo Clin Proc 83(3):355–367
Morgensztern D, Ng SH, Gao F et al (2010) Trends in stage distribution for patients with non-small cell lung cancer: a National Cancer Database survey. J Thorac Oncol 5(1):29–33
König D, Savic Prince S, Rothschild SI (2021) Targeted therapy in Advanced and Metastatic Non-Small Cell Lung Cancer. An update on treatment of the most important actionable oncogenic driver alterations. Cancers (Basel) 13(4):804
Baggstrom MQ, Stinchcombe TE, Fried DB et al (2007) Third-generation chemotherapy agents in the treatment of advanced non-small cell lung cancer: a meta-analysis. J Thorac Oncol 2(9):845–853
Reck M, Rodríguez-Abreu D, Robinson AG et al (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-cell Lung Cancer. N Engl J Med 375(19):1823–1833
Mok TSK, Wu YL, Kudaba I et al (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 393(10183):1819–1830
Herbst RS, Giaccone G, de Marinis F et al (2020) Atezolizumab for First-Line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med 383(14):1328–1339
Gandhi L, Rodríguez-Abreu D, Gadgeel S et al (2018) Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 378(22):2078–2092
Paz-Ares L, Luft A, Vicente D et al (2018) Pembrolizumab plus Chemotherapy for squamous non-small-cell Lung Cancer. N Engl J Med 379(21):2040–2051
Socinski MA, Jotte RM, Cappuzzo F et al (2018) Atezolizumab for First-Line treatment of metastatic nonsquamous NSCLC. N Engl J Med 378(24):2288–2301
Jotte R, Cappuzzo F, Vynnychenko I et al (2020) Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced squamous NSCLC (IMpower131): results from a Randomized Phase III Trial. J Thorac Oncol 15(8):1351–1360
Liao W, Lin JX, Leonard WJ (2013) Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38(1):13–25
Malek TR, Castro I (2010) Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 33(2):153–165
Heaney ML, Golde DW (1998) Soluble receptors in human disease. J Leukoc Biol 64(2):135–146
Dejica D (2001) Serum soluble IL-2 receptor as a marker of lymphocyte activation in some autoimmune diseases. Effect of immunosuppressive therapy. Roum Arch Microbiol Immunol 60(3):183–201
Correia O, Delgado L, Roujeau JC et al (2002) Soluble interleukin 2 receptor and interleukin 1alpha in toxic epidermal necrolysis: a comparative analysis of serum and blister fluid samples. Arch Dermatol 138(1):29–32
Gundlach E, Hoffmann MM, Prasse A et al (2016) Interleukin-2 receptor and angiotensin-converting enzyme as markers for ocular sarcoidosis. PLoS ONE 11(1):e0147258
Cabrera R, Ararat M, Cao M et al (2010) Hepatocellular carcinoma immunopathogenesis: clinical evidence for global T cell defects and an immunomodulatory role for soluble CD25 (sCD25). Dig Dis Sci 55(2):484–495
Lindqvist CA, Christiansson LH, Simonsson B et al (2010) T regulatory cells control T-cell proliferation partly by the release of soluble CD25 in patients with B-cell malignancies. Immunology 131(3):371–376
Pedersen AE, Lauritsen JP (2009) CD25 shedding by human natural occurring CD4 + CD25 + regulatory T cells does not inhibit the action of IL-2. Scand J Immunol 70(1):40–43
Diem S, Schmid S, Krapf M et al (2017) Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 111:176–181
Adachi Y, Tamiya A, Taniguchi Y et al (2020) Predictive factors for progression-free survival in non-small cell lung cancer patients receiving nivolumab based on performance status. Cancer Med 9(4):1383–1391
Jiang M, Peng W, Pu X et al (2020) Peripheral blood biomarkers Associated with Outcome in Non-small Cell Lung Cancer Patients treated with Nivolumab and Durvalumab Monotherapy. Front Oncol 10:913
Agulló-Ortuño MT, Gómez-Martín Ó, Ponce S et al (2020) Blood predictive biomarkers for patients with non-small-cell Lung Cancer Associated with Clinical Response to Nivolumab. Clin Lung Cancer 21(1):75–85
Kazandjian D, Gong Y, Keegan P et al (2019) Prognostic value of the lung Immune Prognostic Index for Patients treated for metastatic non-small cell Lung Cancer. JAMA Oncol 5(10):1481–1485
Kanda Y (2013) Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 48(3):452–458
Fehniger TA, Cooper MA, Caligiuri MA (2002) Interleukin-2 and interleukin-15: immunotherapy for cancer. Cytokine Growth Factor Rev 13(2):169–183
Rubin LA, Kurman CC, Fritz ME et al (1985) Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol 135(5):3172–3177
Bien E, Balcerska A (2008) Serum soluble interleukin 2 receptor alpha in human cancer of adults and children: a review. Biomarkers 13(1):1–26
Burton J, Kay NE (1994) Does IL-2 receptor expression and secretion in chronic B-cell leukemia have a role in down-regulation of the immune system? Leukemia 8(1):92–96
Yang ZZ, Grote DM, Ziesmer SC et al (2011) Soluble IL-2Rα facilitates IL-2-mediated immune responses and predicts reduced survival in follicular B-cell non-hodgkin lymphoma. Blood 118(10):2809–2820
Cabrera R, Ararat M, Eksioglu EA et al (2010) Influence of serum and soluble CD25 (sCD25) on regulatory and effector T-cell function in hepatocellular carcinoma. Scand J Immunol 72(4):293–301
Waldmann TA (1990) The multichain interleukin 2 receptor. A target for immunotherapy in lymphoma, autoimmune disorders, and organ allografts [clinical reference]. JAMA 263(2):272–274
Jobin N, Garrel D, Bernier J (2000) Increased serum-soluble interleukin-2 receptor in burn patients: characterization and effects on the immune system. Hum Immunol 61(3):233–246
Ennishi D, Yokoyama M, Terui Y et al (2009) Soluble interleukin-2 receptor retains prognostic value in patients with diffuse large B-cell lymphoma receiving rituximab plus CHOP (RCHOP) therapy. Ann Oncol 20(3):526–533
Gupta M, Stenson M, O’Byrne M et al (2016) Comprehensive serum cytokine analysis identifies IL-1RA and soluble IL-2Rα as predictors of event-free survival in T-cell lymphoma. Ann Oncol 27(1):165–172
Buccheri G, Marino P, Preatoni A et al (1991) Soluble interleukin 2 receptor in lung cancer. An indirect marker of tumor activity? Chest 99(6):1433–1437
Lissoni P, Barni S, Rovelli F et al (1990) The biological significance of soluble interleukin-2 receptors in solid tumors. Eur J Cancer 26(1):33–36
Hellmann MD, Paz-Ares L, Bernabe Caro R et al (2019) Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med 381(21):2020–2031
Paz-Ares L, Ciuleanu TE, Cobo M et al (2021) First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol 22(2):198–211
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research received no specific grant.
Author information
Authors and Affiliations
Contributions
Study concept and design: TT, SK, MN. Acquisition and analysis of data and statistical analysis: TT, NY, HY, RM, SO, RT, HS, YA, RA, KU, SK, MN. Interpretation of data and drafting of the manuscript: TT, NY, HY, RM, SO, RT, HS, YA, RA, KU, SK, MS, AG, MN. Critical revision of the manuscript for important intellectual content: TT, NY, MS, AG, MN. Study supervision: MN. All authors approved the final version of the manuscript and agreed to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the Ethics Committee of the Cancer Institute Hospital, Japanese Foundation for Cancer Research (approval number 2019 − 1043).
Consent to participate
Written informed consents were obtained from all the patients.
Competing interests
Makoto Nishio received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer-Ingelheim, MSD, Novartis; and received research funding from MSD, Novartis, Ono Pharmaceutical, Chugai Pharmaceutical, Bristol Myers Squibb, Taiho Pharmaceutical, Eli Lilly, AstraZeneca, Pfizer, Astellas; and had consulting/advisory roles for Novartis, Daiichi Sankyo Healthcare, Taiho Pharmaceutical, Bristol Myers Squibb, Boehringer-ingelheim, Ono Pharmaceutical, Eli Lilly, Chugai Pharmaceutical, AstraZeneca, Merck Serono, MSD, Pfizer. Akihiko Gemma has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Chugai Pharmaceutical, Taiho Pharmaceutical, Daiichi Sankyo Healthcare, KYORIN Pharmaceutical, Accuray Japan, Boehringer-ingelheim, MSD, AstraZeneca, Kyowa Kirin, Otsuka Pharmaceutical, Eisai; and received research funding from Nippon Kayaku. Masahiro Seike has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, Taiho Pharmaceutical, AstraZeneca, Boehringer-ingelheim, MSD; and received research funding from Taiho Pharmaceutical, Chugai Pharmaceutical, Boehringer-ingelheim. Noriko Yanagitani has received honoraria from Ono Pharmaceutical, Taiho Pharmaceutical, MSD, Novartis, Bayer Yakuhin, and had consulting/advisory roles for Chugai Pharmaceutical. Ken Uchibori has received honoraria from Ono Pharmaceutical, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly, AstraZeneca, Boehringer-ingelheim, and had consulting/advisory roles for AstraZeneca, Chugai Pharmaceutical. Satoru Kitazono has received honoraria from Chugai Pharmaceutical Co., Ltd., MSD K.K., Bristol-Myers Squibb, ONO Pharmaceutical, AstraZeneca. All the other authors report no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tozuka, T., Yanagitani, N., Yoshida, H. et al. Soluble interleukin-2 receptor as a predictive biomarker for poor efficacy of combination treatment with anti-PD-1/PD-L1 antibodies and chemotherapy in non-small cell lung cancer patients. Invest New Drugs 41, 411–420 (2023). https://doi.org/10.1007/s10637-023-01358-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-023-01358-3